Efficient Analysis of Kava Extract: New phytochemical certified reference material mixes for ginger and kava
Adam J. Kuszak; Stephen A. Wise1, Chris Leija, Senior Scientist, Reference Materials & Workflows2, Uma Sreenivasan, Head of Reference Materials & Workflows R&D2, Matthias Nold, Product Manager Reference Materials2
1Office of Dietary Supplements, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland, USA, 2MilliporeSigma

Introduction
Plant-based products are widely used for herbal medicinal drugs, dietary supplements, or cosmetic applications. The complexity and the natural variations in the chemical composition of plants pose big challenges to quality control and authenticity confirmation of botanical products. To verify the plant species and detect potential adulteration with different plant parts, different plant species, or the addition of synthetic components, chromatographic fingerprint analysis of the most common chemical markers is an efficient method. We have a comprehensive portfolio of more than 1500 phytochemical neat standards of the most important marker compounds for a large variety of plants.
Quantitative and qualitative analysis of botanical materials, usually requires testing of multiple key components. Analysis requires time-consuming preparation of multi-component solutions from the neat materials. The stability of the solutions must be established, possibly requiring frequent preparations. To offer an effective alternative, we supply a series of plant-specific Certified Reference Material (CRM) mixes. These Supelco® branded products, produced at the Cerilliant® site in Round Rock Texas under ISO/IEC 17025 and ISO 17034 accreditation, offer a cost- efficient and precise tool for calibration and verification of multiple key components for a series of popular medicinal plants and dietary supplements. The CRMs are supplied with a comprehensive certificate of analysis, including traceable values and expanded uncertainties for all the analytes.
A new kava mix has recently been added to this portfolio (composition shown in Table 1). This new product was developed in collaboration with the U.S. National Institutes of Health (NIH) Office of Dietary Supplements (ODS) along with other products in development (e.g. a CRM mix for gingerols and shaogols from ginger). Responding to the increased biomedical interest in kava’s health effects and safety, as well as an expressed need to more rigorously characterize food and dietary supplement preparations1, the ODS Analytical Methods and Reference Materials Program contracted with Cerilliant® corporation to develop a CRM calibration solution of major kava constituents to aid industry, academic, and regulatory scientists and analysts.
Background
The kava plant, also called kava kava (Piper methysticum G. Forst.), has its origins in the islands of the South Pacific and is increasingly popular as an herbal medicine and dietary supplement in other parts of the world. Traditionally consumed as a ceremonial beverage prepared from the rhizomes, kava also has a long history of use in traditional medicines for its relaxing effects.2 Kava contains numerous kavalactones and flavokawains (also referred to as flavokavains) which may have unique and/or synergistic bioactivities, and different kava cultivars can contain varying proportions of these constituents. Current biomedical research is investigating the safety and efficacy of kava constituents for treating anxiety disorders and their potential in preventing or modulating cancer progression.3-5 The early 2000’s saw a number of cases of hepatotoxicity associated with kava consumption, causing public health concerns and leading to import restrictions in several countries6, though many regulatory controls have recently been eased. Some research suggests kava’s potential for hepatotoxicity may be related to the types of solvents used in commercial extracts or due to herb-drug interactions with specific flavokawains. However, an understanding of the safety of kava's constituents continues to be investigated.
Sample Preparation and Analytical Method
Analysis of samples of ground kava root (noble cultivar) as well as capsules containing Kava Kava extract was performed. For the quantitative and qualitative evaluation, the samples were compared with the new Kava CRM Mix. Figure 1 shows the chemical structure of the kavalactones and flavokavains included in the CRM Mix cat.no. K-007. Using the Kava CRM solution, a 5-point calibration curve was constructed spanning 6.25 – 250 µg/mL for the kavalactones and 0.625 – 25 µg/mL for the flavokavains.
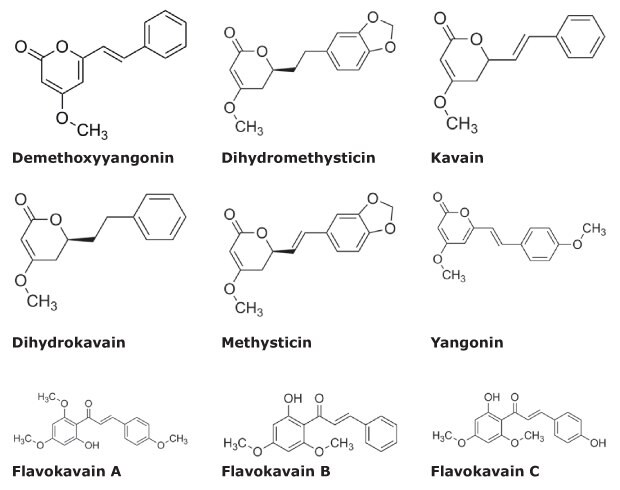
Figure 1.Chemical Structures of Kava Ingredients.
For the analysis, 750 mg of kava root or kava capsules respectively was suspended in 50 mL extraction solvent consisting of a 70:30 mixture of methanol and water and sonicated for 60 min. After centrifugation at 4000 rpm for 5 minutes, the soluble fraction was passed through a 0.2 µm PTFE syringe filter and injected into the HPLC (conditions Table 2).
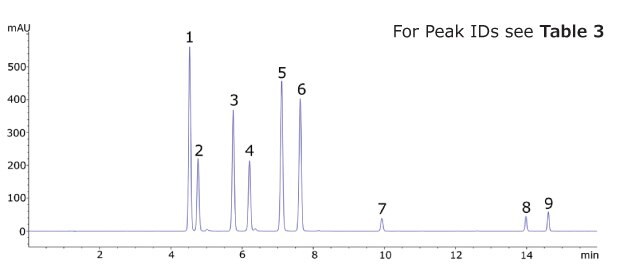
Figure 2.Chromatogram of the Kava CRM Mix Cat.No. K-007.
Results
The Ascentis® Express C18 column and kava CRM solution were used to successfully separate and quantitate kavalactones and flavokavains in two types of kava products. Single component solutions of each analyte were prepared for peak identification and determination of elution order. The analysed kava root extract, as well as the kava capsules showed a typical distribution of kavalactones (Figure 3 and 4).
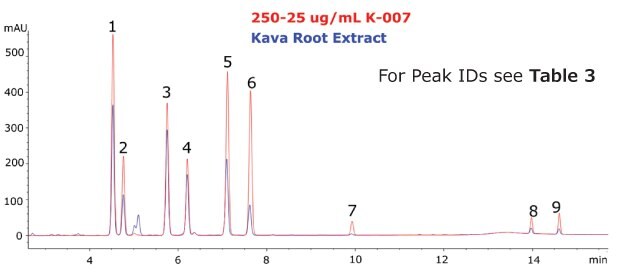
Figure 3.Chromatogram of the Kava CRM Mix Cat.No. K-007 (red) and Kava root extract (blue).
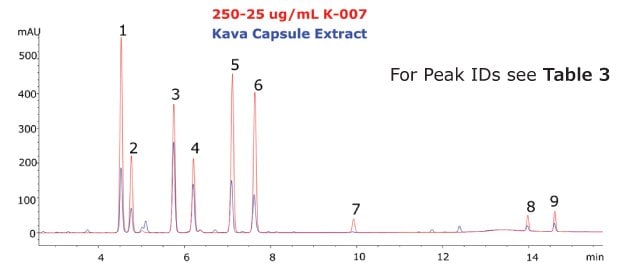
Figure 4.Chromatogram of the Kava CRM Mix Cat.No. K-007 (red) and Kava capsule extract (blue).
The calibration curve demonstrated excellent linearity across the analyte ranges (R2 > 0.999). Quantitation of each analyte revealed a low abundance of flavokavains relative to kavalactones in both samples. The total sum (% weight basis) of kavalactones and flavokavains in the root and capsule samples was found to be 5.68% and 17.90%, respectively (Table 3).
Conclusion
The results of this analysis demonstrate that the Kava CRM solution can easily be adopted for use in HPLC- UV analysis of kavalactones and flavokavains in kava products. The use of kava CRMs in a solution format provides the convenience and efficiency of material usage, consistency, accuracy, and metrological traceability. The new kava CRM mix is a very efficient tool to analyse the chemical compositions of kava products to confirm their potency and authenticity.
An overview on our phytochemical mixes can be found at SigmaAldrich.com/phytochemicalmixes
References
Um weiterzulesen, melden Sie sich bitte an oder erstellen ein Konto.
Sie haben kein Konto?