93744
Bromopropylate
certified reference material, TraceCERT®, Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
Synonym(s):
Isopropyl 4,4′-dibromobenzilate, Phenisobromolate
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
Quality Level
product line
TraceCERT®
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
Manufactured by: Sigma-Aldrich Production GmbH, Switzerland
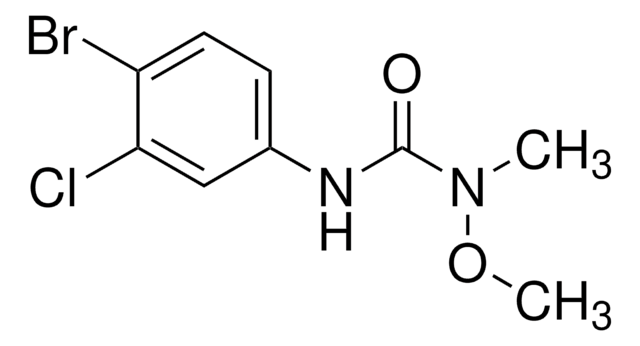
SMILES string
CC(C)OC(=O)C(O)(c1ccc(Br)cc1)c2ccc(Br)cc2
InChI
1S/C17H16Br2O3/c1-11(2)22-16(20)17(21,12-3-7-14(18)8-4-12)13-5-9-15(19)10-6-13/h3-11,21H,1-2H3
InChI key
FOANIXZHAMJWOI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Certified content by quantitative NMR incl. uncertainty and expiry date are given on the certificate.
Download your certificate at: http://www.sigma-aldrich.com
Bromopropylate is a nonsystemic and nonpenetrating acaricide that remains on the peel of fruits and does not migrate into the pulp. It belongs to the family of organophosphate pesticides and is used in the cultivation of lemon trees and other crops. Bromopropylate primarily acts by interfering with the respiratory system. It affects the mitochondria by inhibiting key enzymatic activities and ATP generation, which leads to the formation of reactive oxygen species, and destroys proteins, membranes, unsaturated fatty acids, DNA, etc.
Bromopropylate has to be monitored in the Multiannual Control Programme for Pesticides Residues (MACP), run within the EU and EFTA in/on products of plant origin.
The use of bromopropylate has been banned across the European Union. Maximum residue levels (MRLs) have been set according to Reg. (EU) No 310/2011 for bromopropylate for various products of plant and animal origin from 0.01 to 0.05 mg/kg.
Application
- To elucidate the inhibition of plasma and brain acetylcholinesterase activity in Wistar rats with a mixture of 5 pesticides: chlorpyrifos, alphacypermetrin, bromopropylate, carbendazim, and mancozeb
- Study the degradation processes of bromopropylate, coumaphos, chlordimeform, cymiazole, flumethrin, and fluvalinate in aqueous media by HPLC
- To develop a liquid-phase microextraction method using deep eutectic solvent for extraction and preconcentration of diazinon, metalaxyl, bromopropylate, oxadiazon, and fenazaquin pesticides from samples such as grape and sour cherry juices, fresh beet, cucumber, potato, and tomato followed by gas chromatography-flame ionization detection
- To develop a multi-residue method for the determination of azinphos methyl, bromopropylate, chlorpyrifos, dimethoate, parathion methyl, and phosalone in apricots and peaches using negative chemical ionization ion trap technology
- Simultaneous determination of amitraz, bromopropylate, coumaphos, cymiazole, and fluvalinate residues in honey by solid-phase extraction and GC-MS
Recommended products
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service