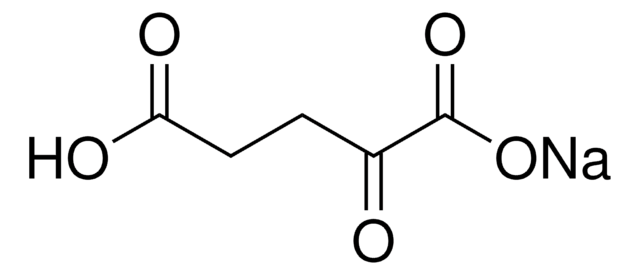
K1502
α-Ketoglutarate Dehydrogenase from porcine heart
buffered aqueous glycerol solution, 0.1-1.0 units/mg protein (Lowry)
Synonym(s):
Multienzyme 2-oxoglutarate dehydrogenase complex
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
buffered aqueous glycerol solution
specific activity
0.1-1.0 units/mg protein (Lowry)
foreign activity
pyruvate dehydrogenase ≤20%
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Research Area: Neuroscience
α-Ketoglutarate dehydrogenase (α-KGDH) is a multienzyme complex localized to the mitochondria. This integrated enzyme is made up of many units of thiamine pyrophosphate-dependent dehydrogenase (E1), dihydrolipoamide dehydrogenase (E3), and dihydrolipoamide succinyl transferase (E2).
α-Ketoglutarate dehydrogenase (α-KGDH) is a multienzyme complex localized to the mitochondria. This integrated enzyme is made up of many units of thiamine pyrophosphate-dependent dehydrogenase (E1), dihydrolipoamide dehydrogenase (E3), and dihydrolipoamide succinyl transferase (E2).
Application
α-Ketoglutarate Dehydrogenase from the porcine heart has been used:
- to study the reversal of nitration by glutathione (GSH) in peroxynitrite-treated cells
- to measure its activity by Spectramax M5 microplate spectrofluorimeter using heart mitochondria
- as a positive control to evaluate its activity in by Spectramax GEMINI EM fluorescence microplate reader using mice neurons
Biochem/physiol Actions
α-Ketoglutarate dehydrogenase (α-KGDH) is a key enzyme of bioenergetic processes and a controlling unit of metabolic flux through the Krebs cycle or tricarboxylic acid (TCA) cycle. It catalyzes the oxidative decarboxylation of α-ketoglutarate (KG) to succinyl-CoA by releasing reduced nicotinamide adenine dinucleotide (NADH). It is the rate-limiting reaction of the TCA cycle. This reaction contributes to the electrons of the respiratory chain and requires thiamine pyrophosphate as a cofactor. The reduction of NAD (nicotinamide adenine dinucleotide) is observed to determine its reaction rate. α-KGDH from porcine has an optimum pH range of 6.6–7.4. This enzyme is inhibited by oxidative stress and results in a metabolic deficiency. However, α-KGDH is also known to produce reactive oxygen species (ROS) leading to oxidative stress. Defective or limited levels of α-KGDH cause several neurodegenerative diseases such as Alzheimer′s disease.
α-Ketoglutarate dehydrogenase is most responsive to alterations in the tumor microenvironment and contributes to the adaptive metabolic response in cancer. Inhibiting α-ketoglutarate dehydrogenase counteracts lung metastasis associated with breast cancer.
Quality
May contain traces of polyethylene glycol.
Unit Definition
One unit will convert 1.0 μmole of β-NAD to β-NADH per min at pH 7.4 at 30 °C in the presence of saturating levels of coenzyme A.
Physical form
Supplied as a 50% glycerol solution containing ~9 mg per mL bovine serum albumin, 30% sucrose, 1.5 mM EDTA, 1.5 mM EGTA, 1.5 mM 2-mercaptoethanol, 0.3% TRITON™ X-100, 0.003% sodium azide, and 15 mM potassium phosphate, pH 6.8.
Legal Information
Triton is a trademark of The Dow Chemical Company or an affiliated company of Dow
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
?-ketoglutarate dehydrogenase from pig heart
Test, 13, 52-55 (1969)
Dirk Steinhauser et al.
Trends in plant science, 17(9), 503-509 (2012-06-05)
As a fundamental energy-conserving process common to all living organisms, respiration is responsible for the oxidation of respiratory substrates to drive ATP synthesis. Accordingly, it has long been accepted that a complete tricarboxylic acid (TCA) cycle is necessary for respiratory
Gary E Gibson et al.
Journal of neurochemistry, 134(1), 86-96 (2015-03-17)
Reversible post-translation modifications of proteins are common in all cells and appear to regulate many processes. Nevertheless, the enzyme(s) responsible for the alterations and the significance of the modification are largely unknown. Succinylation of proteins occurs and causes large changes
alpha-ketoglutarate dehydrogenase inhibition counteracts breast cancer-associated lung metastasis
Atlante S, et al.
Cell Death & Disease, 9(7), 756-756 (2018)
Shuyi Zhang et al.
Science (New York, N.Y.), 334(6062), 1551-1553 (2011-12-17)
It is generally accepted that cyanobacteria have an incomplete tricarboxylic acid (TCA) cycle because they lack 2-oxoglutarate dehydrogenase and thus cannot convert 2-oxoglutarate to succinyl-coenzyme A (CoA). Genes encoding a novel 2-oxoglutarate decarboxylase and succinic semialdehyde dehydrogenase were identified in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service