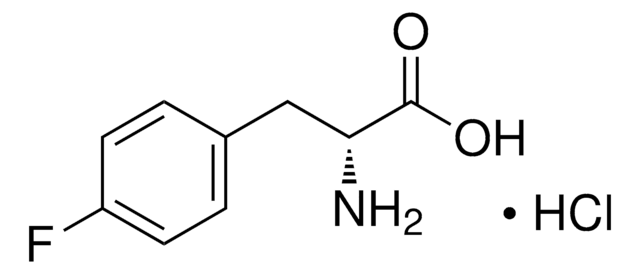
F5126
m-Fluoro-DL-phenylalanine
≥98%
Synonym(s):
3-Fluoro-DL-phenylalanine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4CH2CH(NH2)COOH
CAS Number:
Molecular Weight:
183.18
Beilstein:
2939807
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
m-Fluoro-DL-phenylalanine,
Assay
≥98%
Quality Level
form
powder
color
white to off-white
mp
240-250 °C
application(s)
cell analysis
peptide synthesis
SMILES string
NC(Cc1cccc(F)c1)C(O)=O
InChI
1S/C9H10FNO2/c10-7-3-1-2-6(4-7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
VWHRYODZTDMVSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
m-Fluoro-DL-phenylalanine, a toxic antimetabolite, is a racemic mixture of a substituted (halogenated) benzoyl D and L phenylalanine with potential use in antiviral and antimicrobial drug development.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nick J P Wierckx et al.
Applied and environmental microbiology, 71(12), 8221-8227 (2005-12-08)
Efficient bioconversion of glucose to phenol via the central metabolite tyrosine was achieved in the solvent-tolerant strain Pseudomonas putida S12. The tpl gene from Pantoea agglomerans, encoding tyrosine phenol lyase, was introduced into P. putida S12 to enable phenol production.
M P Gamcsik et al.
FEBS letters, 196(1), 71-74 (1986-02-03)
Rabbits ingesting 4-fluorophenylalanine are known to incorporate small amounts of this fluorinated amino acid into their proteins. Carbonic anhydrase I isolated from the erythrocytes of animals so maintained exhibits a well-resolved fluorine NMR signal for each phenylalanine in the sequence.
D H Young et al.
Experientia, 45(4), 325-327 (1989-04-15)
The tripeptide L-m-fluorophenylalanyl-L-alanyl-L-alanine was much more fungitoxic towards Pythium ultimum than the dipeptide L-m-fluorophenylalanyl-L-alanine or m-fluorophenylalanine. The fungitoxicity of the tripeptide was reduced by L-alanyl peptides and phenylalanine, but not by other amino acids. In contrast, the fungitoxicity of m-fluorophenylalanine
Jennifer C Jackson et al.
Journal of the American Chemical Society, 129(5), 1160-1166 (2007-02-01)
19F NMR is a powerful tool for monitoring protein conformational changes and interactions; however, the inability to site-specifically introduce fluorine labels into proteins of biological interest severely limits its applicability. Using methods for genetically directing incorporation of unnatural amino acids
H D Dettman et al.
Biophysical journal, 37(1), 243-251 (1982-01-01)
The nonlytic, filamentous coliphage M13 offers an excellent model system for the study of membrane-protein interactions. We prepare derivatives of the protein containing fluorine-labeled amino acids and use 19F nuclear magnetic resonance (NMR) to study the protein in both deoxycholate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service