8.56044
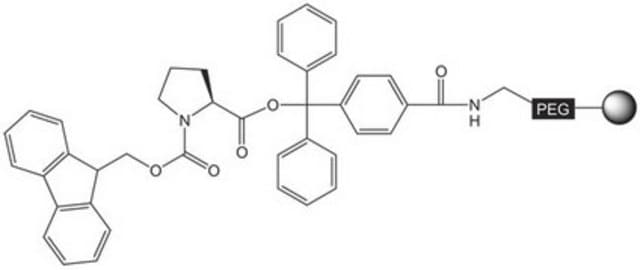
Fmoc-Cys(Trt)-NovaSyn® TGT
for peptide synthesis, Novabiochem®
Synonym(s):
Trityl-Protected Cysteine Resin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
product name
Fmoc-Cys(Trt)-NovaSyn® TGT, Novabiochem®
Quality Level
product line
NovaSyn® TG
Novabiochem®
form
solid
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
15-25°C
General description
Pre-loaded resin for synthesis of peptide acids and protected peptide fragments containing a C-terminal cysteine amino-acid residue by Fmoc SPPS. The base NovaSyn® TG is a composite of low cross-linked polystyrene and 3000-4000 M.W. polyethylene glycol. Peptide synthesis is carried out at the ends of the PEG chains that have been functionalized with the hyper-acid labile 4-carboxytrityl alcohol linker. This use of this linker helps prevent racemization and β-piperidinylalanine formation during chain extension.Treatment of the peptidyl resin with 20% TFE in DCM or 1% TFA in DCM cleaves the product from the resin without affecting the standard TFA-labile side-chain protecting groups. Standard TFA cleavage releases the fully deprotected peptide.
Associated Protocols and Technical Articles:
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] E. Atherton, et al. in "Peptides 1990, Proc. 21st European Peptide Symposium, E. Giralt & D. Andreu(Eds), 1991, Escom, Leiden, pp. 243
[2] J. Lukszo, et al. (1996) Lett. Pept. Sci., 3, 157.
[3] Y. Fujiwara, et al. (1994) Chem. Pharm. Bull., 42, 724.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Associated Protocols and Technical Articles:
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] E. Atherton, et al. in "Peptides 1990, Proc. 21st European Peptide Symposium, E. Giralt & D. Andreu(Eds), 1991, Escom, Leiden, pp. 243
[2] J. Lukszo, et al. (1996) Lett. Pept. Sci., 3, 157.
[3] Y. Fujiwara, et al. (1994) Chem. Pharm. Bull., 42, 724.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Application
Applications of Fmoc-Cys(Trt)-NovaSyn® TGT include:
- the preparation of a phosphoprotein using Native Chemical Ligation (NCL).
- the preparation of a peptide intermediate for use in glycoprotein semi-synthesis.
Linkage
Replaces: 04-12-2705
Legal Information
NOVASYN is a registered trademark of Merck KGaA, Darmstadt, Germany
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K. Barlos & D. Gatos in ?Fmoc solid phase peptide synthesis: a practical approach?, W. C. Chan & P. D.
White (Eds.), Oxford University Press, Oxford
K. Barlos & D. Gatos
Solid Phase Peptide Synthesis: A Practical Approach, 218-218 (2000)
3-(1-Piperidinyl) alanine formation during the preparation of C-terminal cysteine peptides with the Fmoc/t-Bu strategy
J. Lukszo, et al.
Peptide science (Hoboken, N.J.), 3, 157-157 (1996)
Peptides 1990, Proc. 21st European Peptide Symposium, E. Giralt & D. Andreu
E. Atherton, et al. in
Proceedings, 243-243 (1991)
Access to phosphoproteins and glycoproteins through semi-synthesis, Native Chemical Ligation and N? S acyl transfer
Masania J, et al.
Organic & Biomolecular Chemistry, 5113-5119 (2010)
Racemization-free synthesis of C-terminal cysteine-peptide using 2-chlorotrityl resin
Y. Fujiwara, et al.
Journal of Chemical and Pharmaceutical Sciences
, 42, 724-724 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service