C85603
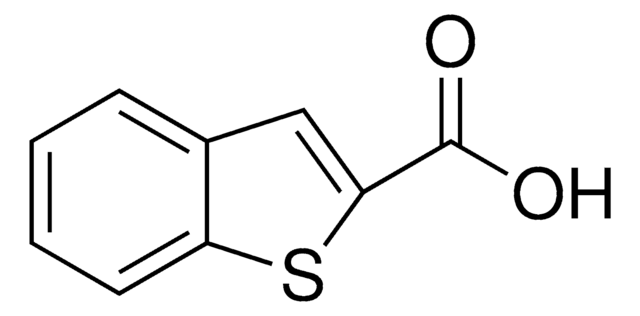
Coumarin-3-carboxylic acid
99%
Synonym(s):
2-Oxo-2H-1-benzopyran-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H6O4
CAS Number:
Molecular Weight:
190.15
Beilstein:
154276
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
189-192 °C (lit.)
SMILES string
OC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C10H6O4/c11-9(12)7-5-6-3-1-2-4-8(6)14-10(7)13/h1-5H,(H,11,12)
InChI key
ACMLKANOGIVEPB-UHFFFAOYSA-N
Gene Information
human ... PTPN1(5770)
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amarjit Singh et al.
International journal of radiation biology, 84(12), 1001-1010 (2008-12-09)
To synthesize N-(3-(3-aminopropylamino)propyl)-2-oxo-2H-chromene-3-carboxamide (7), a novel DNA-binding, coumarin-based, fluorescent hydroxylradical ((*)OH) indicator and to assess its quantum efficiency compared with that of coumarin-3-carboxylic acid (1) and N1,N12-bis[2-oxo-2H-chromene-3-carbonyl]- 1,12-diamine-4,9-diazadodecane (9). Using computer-generated molecular modeling, 7 and 9 and their respective 7-hydroxylated
A Karaliota et al.
Journal of inorganic biochemistry, 84(1-2), 33-37 (2001-05-02)
The copper(II) complex of coumarin-3-carboxylic acid (CcaH) has been prepared and characterized on the basis of elemental and thermal analysis, IR, Raman, EPR, UV-Vis reflectance and 1H-NMR spectra. A detail analysis of all spectra data is presented with particular emphasis
Judith A K Howard et al.
Acta crystallographica. Section B, Structural science, 65(Pt 2), 230-237 (2009-03-21)
As part of an ongoing series of experimental charge-density investigations into the intra- and intermolecular interactions present in compounds which undergo solid-state [2 + 2] cycloaddition reactions, the charge-density analyses of trans-cinnamic acid and coumarin-3-carboxylic acid are reported. Thus, high-resolution
Bhumika Thati et al.
Cancer letters, 248(2), 321-331 (2006-09-26)
The chemotherapeutic potential of coumarin-3-carboxylic acid (C-3-COOH) and a series of three hydroxylated coumarin-3-carboxylic acid ligands, namely 6-hydroxy-coumarin-3-carboxylic acid (6-OH-C-3-COOH), 7-hydroxy-coumarin-3-carboxylic acid (7-OH-C-3-COOH) and 8-hydroxy-coumarin-3-carboxylic acid (8-OH-C-3-COOH), along with their corresponding silver-based complexes, namely 6-hydroxycoumarin-3-carboxylatosilver (6-OH-C-COO-Ag), 7-hydroxycoumarin-3-carboxylatosilver (7-OH-C-COO-Ag) and 8-hydroxycoumarin-3-carboxylatosilver
Gertjan J M den Hartog et al.
Chemico-biological interactions, 145(1), 33-39 (2003-02-28)
Cu,Zn-superoxide dismutase (SOD1) has been shown to be effective in several free radical mediated diseases, although some studies have pointed toward SOD1 toxicity at a high concentrations. In the present study, the balance between prevention and induction of damage by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service