All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H2Cl3N
CAS Number:
Molecular Weight:
182.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
75-77 °C (dec.) (lit.)
functional group
chloro
SMILES string
Clc1cncc(Cl)c1Cl
InChI
1S/C5H2Cl3N/c6-3-1-9-2-4(7)5(3)8/h1-2H
InChI key
KKWRVUBDCJQHBZ-UHFFFAOYSA-N
General description
3,4,5-Trichloropyridine is a pyridine derivative. It undergoes oxidation with trifluoroacetic anhydride (TFAA) in the presence of hydrogen peroxide urea complex.
Application
3,4,5-Trichloropyridine may be employed as a secondary standard in quantitative NMR (qNMR) studies.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A practical, efficient, and rapid method for the oxidation of electron deficient pyridines using trifluoroacetic anhydride and hydrogen peroxide-urea complex.
Caron S, et al.
Tetrahedron Letters, 41(14), 2299-2302 (2000)
Torgny Rundlöf et al.
Journal of pharmaceutical and biomedical analysis, 52(5), 645-651 (2010-03-09)
In quantitative NMR (qNMR) selection of an appropriate internal standard proves to be crucial. In this study, 25 candidate compounds considered to be potent internal standards were investigated with respect to the ability of providing unique signal chemical shifts, purity
Torgny Rundlöf et al.
Journal of pharmaceutical and biomedical analysis, 93, 111-117 (2013-11-12)
Standards are required in quantitative NMR (qNMR) to obtain accurate and precise results. In this study acetanilide was established and used as a primary standard. Six other chemicals were selected as secondary standards: 3,4,5-trichloropyridine, dimethylterephthalate, maleic acid, 3-sulfolene, 1,4-bis(trimethylsilyl)benzene, and
Shi Shen et al.
Journal of pharmaceutical and biomedical analysis, 114, 190-199 (2015-06-13)
This study investigated the accuracy of the quantitative NMR method for purity determination of ACE inhibitors reference standards and the discovery of two pairs of new diastereoisomers. Six types of ACE inhibitors, imidapril hydrochloride, benazepril hydrochloride, lisinopril, enalapril maleate, quinapril
Monika Johansson et al.
Journal of pharmaceutical and biomedical analysis, 100, 215-229 (2014-08-30)
An effective screening procedure to identify and quantify active pharmaceutical substances in suspected illegal medicinal products is described. The analytical platform, consisting of accurate mass determination with liquid chromatography time-of-flight mass spectrometry (LC-QTOF-MS) in combination with nuclear magnetic resonance (NMR)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service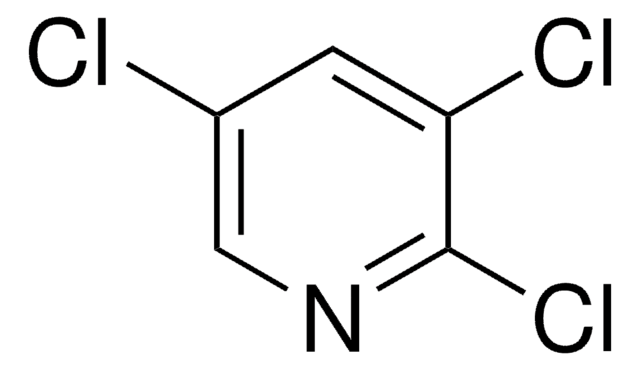






![4,4'-diamino[1,1'-biphenyl]-2,2'-disulfonic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/132/504/f9fd296f-c246-427d-9118-23f7b80e2be1/640/f9fd296f-c246-427d-9118-23f7b80e2be1.png)