All Photos(1)
About This Item
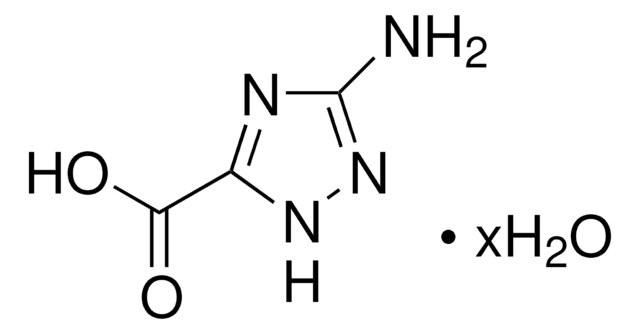
Empirical Formula (Hill Notation):
C11H12N2O3
CAS Number:
Molecular Weight:
220.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
194-195 °C (lit.)
functional group
carboxylic acid
SMILES string
CN1[C@@H]([C@H](CC1=O)C(O)=O)c2cccnc2
InChI
1S/C11H12N2O3/c1-13-9(14)5-8(11(15)16)10(13)7-3-2-4-12-6-7/h2-4,6,8,10H,5H2,1H3,(H,15,16)/t8-,10+/m0/s1
InChI key
DEYLVDCFTICBTB-WCBMZHEXSA-N
Related Categories
General description
Carboxyl group of trans-4-cotininecarboxylic acid (carboxycotinine) can be used for chemical crosslinking.
Application
trans-4-Cotininecarboxylic acid was used in the preparation of cotinine-conjugated horseradish peroxidase during immunoblot analysis. Anion of trans-4-cotininecarboxylic acid has been employed as pyridyl-carboxylate ligand in the preparation of polymeric copper(II) complex.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The use of trans-4-cotininecarboxylate to construct a polymeric copper (II) azido complex: Hydro (solvo) thermal synthesis, structure and magnetic properties.
Luo F, et al.
Inorgorganica Chimica Acta, 363(14), 4117-4122 (2010)
Hyori Kim et al.
Experimental & molecular medicine, 45, e43-e43 (2013-09-28)
We present a bispecific antibody that recognizes an antigen and a hapten and can be applied to various biological assays, including immunoblotting and immunoprecipitation. In immunoblot analysis of serum, an anti-C5 × anti-cotinine bispecific tandem single-chain variable fragment (scFv)-Fc fusion
Soomin Yoon et al.
Journal of cancer research and clinical oncology, 140(2), 227-233 (2013-12-03)
Cotinine has optimal characteristics as a hapten for pre-targeted radioimmunotherapy (PRIT). This study was performed to evaluate the applicability of cotinine/anti-cotinine antibody to PRIT. We developed and prepared a tandem, single-chain, variable fragment Fc fusion protein [tandem single-chain variable fragment
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service