All Photos(1)
About This Item
Linear Formula:
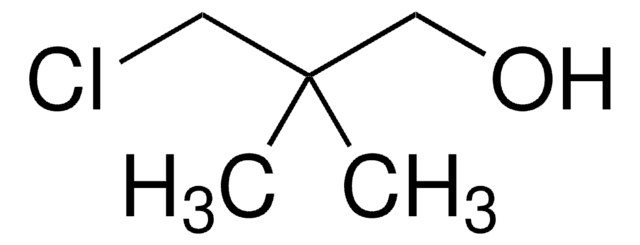
BrCH2C(CH3)2CH2OH
CAS Number:
Molecular Weight:
167.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.479 (lit.)
bp
184-187 °C (lit.)
density
1.358 g/mL at 25 °C (lit.)
functional group
bromo
hydroxyl
SMILES string
CC(C)(CO)CBr
InChI
1S/C5H11BrO/c1-5(2,3-6)4-7/h7H,3-4H2,1-2H3
InChI key
KQOQXYPZBYTICM-UHFFFAOYSA-N
Related Categories
Application
3-Bromo-2,2-dimethyl-1-propanol was used in the synthesis of 3-bromo-2,2-dimethylpropanal by undergoing oxidation with pyridinium chlorochromate, mixed with silica, in dichloromethane.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Erika Leemans et al.
The Journal of organic chemistry, 73(4), 1422-1428 (2008-01-24)
1-Allyl- and 1-(3-phenylallyl)-substituted 4-(2-bromo-1,1-dimethylethyl)azetidin-2-ones were transformed into 3-substituted 7-alkoxy-5,5-dimethyl-1-azabicyclo[4.2.0]octane-8-ones through radical cyclization by means of n-tributyltin hydride and AIBN in toluene with excellent diastereocontrol (>or=99%). The radical cyclization of 4-(2-bromo-1,1-dimethylethyl)-1-(2-methylallyl)azetidin-2-ones afforded 8-alkoxy-3,6,6-trimethyl-1-azabicyclo[5.2.0]nonan-9-ones in good diastereomeric excess (75-78%). The reductive ring
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service