All Photos(1)
About This Item
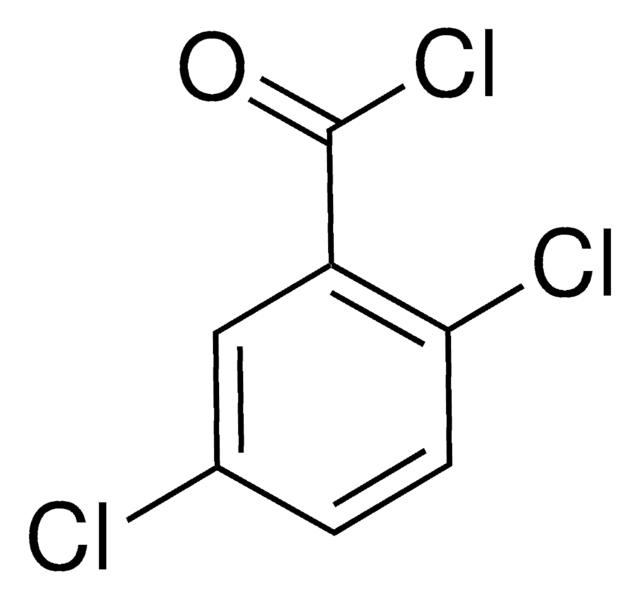
Linear Formula:
Cl2C6H3COCl
CAS Number:
Molecular Weight:
209.46
Beilstein:
639531
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39050513
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.560 (lit.)
bp
142-143 °C/21 mmHg (lit.)
mp
15-17 °C
density
1.462 g/mL at 25 °C (lit.)
functional group
acyl chloride
chloro
SMILES string
ClC(=O)c1c(Cl)cccc1Cl
InChI
1S/C7H3Cl3O/c8-4-2-1-3-5(9)6(4)7(10)11/h1-3H
InChI key
JBLIDPPHFGWTKU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,6-Dichlorobenzoyl chloride participates in esterification of (fluoren-9-ylmethoxy)carbonyl (Fmoc)-amino acids to 4-alkoxybenzyl alcohol polystyrene.
Application
2,6-Dichlorobenzoyl chloride was used:
- in substrate activity screening method for the rapid development of novel substrates and their conversion into non-peptidic inhibitors of Cys and Ser proteases
- in the synthesis of 1-acyliridoles
- in enantiocontrolled total synthesis of (-)-aspicilin
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Morphine recognition by a porphyrin-cyclocholate molecular bowl.
Bonar-Law RP, et al.
Journal of the Chemical Society. Chemical Communications, 5, 456-458 (1993)
An improved method for anchoring of 9-fluorenylmethoxycarbonyl-amino acids to 4-alkoxybenzyl alcohol resins.
Sieber, P.
Tetrahedron Letters, 28(49), 6147-6150 (1987)
Sensitisation to dichlorobenzoyl chloride.
J De Boer et al.
Contact dermatitis, 18(2), 116-117 (1988-02-01)
Andrew W Patterson et al.
Nature protocols, 2(2), 424-433 (2007-04-05)
Substrate activity screening (SAS) is a fragment-based method for the rapid development of novel substrates and their conversion into non-peptidic inhibitors of Cys and Ser proteases. The method consists of three steps: (i) a library of N-acyl aminocoumarins with diverse
Stereocontrolled total synthesis of the macrocyclic lactone (-)-aspicilin.
Waanders PP, et al.
Tetrahedron Letters, 28(21), 2409-2412 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service