134759
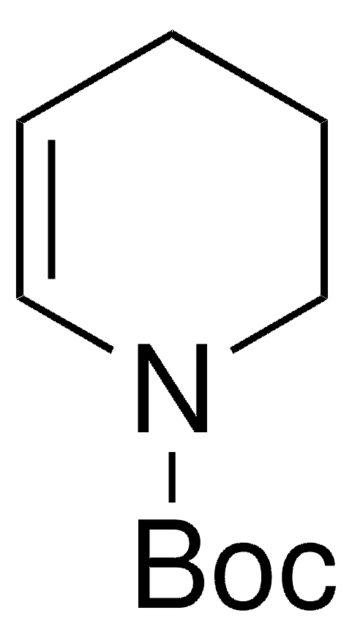
1,2,3,6-Tetrahydropyridine
97%
Synonym(s):
Δ3-Piperidine, 1,2,5,6-Tetrahydropyridine, 3,6-Dihydro-2H-pyridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9N
CAS Number:
Molecular Weight:
83.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.48 (lit.)
bp
108 °C (lit.)
mp
−48 °C (lit.)
density
0.911 g/mL at 25 °C (lit.)
SMILES string
C1CC=CCN1
InChI
1S/C5H9N/c1-2-4-6-5-3-1/h1-2,6H,3-5H2
InChI key
FTAHXMZRJCZXDL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Used to form ene-endo-spirocyclic ammonium ylids for [2,3]-sigmatropic rearrangement to pyrroloazepinones and oxazepinones.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
60.8 °F - closed cup
Flash Point(C)
16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Edward Roberts et al.
Organic letters, 7(10), 2075-2078 (2005-05-07)
The first examples of sigmatropic rearrangements of ene-endo-spirocyclic, tetrahydropyridine-derived ammonium ylids are reported. Thus, spiro[6.7]-ylids rearrange primarily by a [2,3]-pathway, whereas the analogous [6.6]-ylids rearrange by [1,2]- and [2,3]-mechanisms in roughly equal proportions. This method serves as a rapid entry
Santhosh Sethuramanujam et al.
Journal of neurophysiology, 112(1), 193-203 (2014-04-11)
Glutamate release at bipolar to ganglion cell synapses activates NMDA and AMPA/kainic acid (KA) ionotropic glutamate receptors. Their relative strength determines the output signals of the retina. We found that this balance is tightly regulated by presynaptic inhibition that preferentially
Shin'Ichiro Satake et al.
The European journal of neuroscience, 52(3), 3002-3021 (2020-05-10)
Synaptic vesicle exocytosis is triggered by Ca2+ influx through several subtypes of voltage-gated calcium channels in the presynaptic terminal. We previously reported that paired-pulse stimulation at brief intervals increases Cav 2.1 (P/Q-type) channel-mediated multivesicular release (MVR) at glutamatergic synapses between
Cyril G Eleftheriou et al.
Molecular vision, 23, 334-345 (2017-07-01)
Retinal dystrophy through outer photoreceptor cell death affects 1 in 2,500 people worldwide with severe impairment of vision in advanced stages of the disease. Optogenetic strategies to restore visual function to animal models of retinal degeneration by introducing photopigments to
Philippe Huot et al.
Neuropharmacology, 97, 306-311 (2015-06-15)
L-3,4-dihydroxyphenylalanine (L-DOPA) is the most effective anti-parkinsonian agent available, but upon chronic administration, patients with Parkinson's disease (PD) experience abnormal involuntary movements, dyskinesia. Modulation of serotonin 1A (5-HT1A) receptors is regarded as an effective way to alleviate dyskinesia, yet this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service