About This Item
Productos recomendados
assay
≥95%
form
powder
UniProt accession no.
storage temp.
−20°C
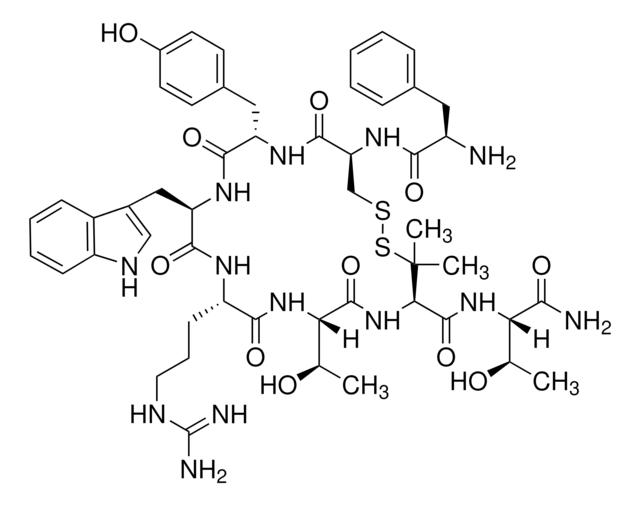
SMILES string
C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc5ccccc5)[C@@H](C)O)C(N)=O
InChI
1S/C51H69N13O11S2/c1-26(65)39(42(53)68)62-49(75)41-51(3,4)77-76-25-38(61-43(69)33(52)21-28-11-6-5-7-12-28)47(73)59-36(22-29-16-18-31(67)19-17-29)45(71)60-37(23-30-24-57-34-14-9-8-13-32(30)34)46(72)58-35(15-10-20-56-50(54)55)44(70)63-40(27(2)66)48(74)64-41/h5-9,11-14,16-19,24,26-27,33,35-41,57,65-67H,10,15,20-23,25,52H2,1-4H3,(H2,53,68)(H,58,72)(H,59,73)(H,60,71)(H,61,69)(H,62,75)(H,63,70)(H,64,74)(H4,54,55,56)/t26-,27-,33-,35+,36+,37-,38+,39+,40+,41-/m1/s1
InChI key
OFMQLVRLOGHAJI-FGHAYEPSSA-N
Gene Information
rat ... Pnoc(25516)
Amino Acid Sequence
Application
- to study the anti-hyperalgesic effect of dipeptidyl peptidase 4 (DPP4) inhibitor isoleucine-proline-isoleucine (IPI) and vildagliptin in carrageenan-induced inflammation
- to study the role of MOR in glutamate and gamma-aminobutyric acid (GABA) efflux during predator stress in rats
- to determine the endogenous opioid peptide involved in blocking pain induced by activated gastrin-releasing peptide (Grp+) neurons
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico