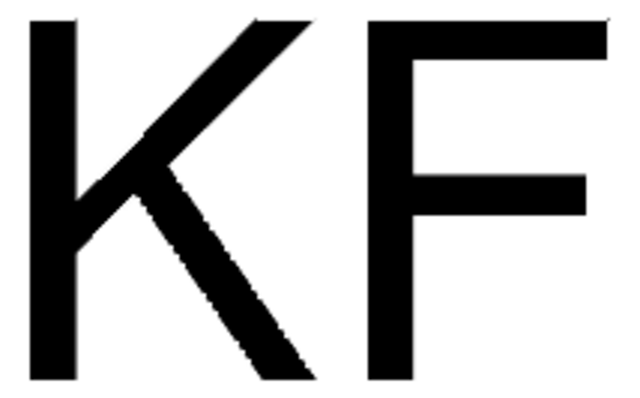939005
Cesium Fluoride ChemBeads

NSC 84270
Sinónimos:
CsF
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
CsF
Número de CAS:
Peso molecular:
151.90
MDL number:
UNSPSC Code:
12352100
Productos recomendados
Quality Level
form
solid
composition
, 14-16 wt. % (loading of base)
SMILES string
[F-].[Cs+]
InChI
1S/Cs.FH/h;1H/q+1;/p-1
InChI key
XJHCXCQVJFPJIK-UHFFFAOYSA-M
General description
Cesium fluoride is an inorganic compound known to be a source of fluoride ion and a catalyst in organic synthesis. It has been used in many organic reactions like 1,4−elimination, desilylation, transesterification, acylation, nucleophilic aromatic substitution, etherification, cross−coupling reactions and so on.
Application
Cesium fluoride can be used as:
A base in the Suzuki cross-coupling synthesis of ortho-substituted biaryls.
A reagent for the nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.
Reactant for:
Preparation of building blocks for synthesis of fluoroallylic compounds
Synthesis of alcohols via hydrolysis of alkyl silyl ethers at neutral pH in buffered mixed organic-aqueous solutions
Nucleophilic fluorination of alkynyliodonium salts to form fluorovinylic compounds
Nucleophilic aromatic substitution (SNAr) reactions
Used in the successful synthesis of a single-crystal Dion-Jacobson phase, CsLaTa2O7, that has applications in photocatalysis and superconductivity.
For general uses, product is also available in powdered form (198323)
A base in the Suzuki cross-coupling synthesis of ortho-substituted biaryls.
A reagent for the nucleophilic fluorination of primary halides and sulfonates in protic media such as tert-butyl and tert-pentyl alcohols.
Reactant for:
Preparation of building blocks for synthesis of fluoroallylic compounds
Synthesis of alcohols via hydrolysis of alkyl silyl ethers at neutral pH in buffered mixed organic-aqueous solutions
Nucleophilic fluorination of alkynyliodonium salts to form fluorovinylic compounds
Nucleophilic aromatic substitution (SNAr) reactions
Used in the successful synthesis of a single-crystal Dion-Jacobson phase, CsLaTa2O7, that has applications in photocatalysis and superconductivity.
For general uses, product is also available in powdered form (198323)
Features and Benefits
ChemBeads are chemical coated glass beads. ChemBeads offer improved flowability and chemical uniformity perfect for automated solid dispensing and high-throughput experimentation. The method of creating ChemBeads uses no other chemicals or surfactants allowing the user to accurately dispense sub-milligram amounts of chemical.
Other Notes
High-Throughput Reaction Screening with Nanomoles of Solid Reagents Coated on Glass Beads
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
related product
Referencia del producto
Descripción
Precios
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Repr. 2 - STOT RE 2
target_organs
Kidney,Adrenal gland
supp_hazards
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificados de análisis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Dong Wook Kim et al.
Journal of the American Chemical Society, 128(50), 16394-16397 (2006-12-15)
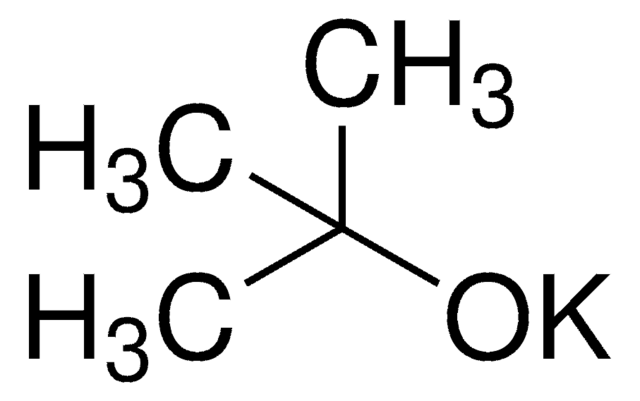
Aprotic solvents are usually preferred for the SN2 reactions, because nucleophilicity and hence SN2 reactivity are severely retarded by the influence of the partial positive charge of protic solvents. In this work, we introduce a remarkable effect of using tertiary
Michael C Willis et al.
Chemical communications (Cambridge, England), (8)(8), 832-833 (2002-07-19)
Treatment of a benzyl substituted meso-ditriflate with boronic acids in the presence of palladium acetate, triphenylphosphine and caesium fluoride results in intermolecular Suzuki coupling followed by vinyl triflate-arene cyclisation to provide, in high yields, single regioisomers of tricyclic-carbocycles.
Takashi Okitsu et al.
Chemical communications (Cambridge, England), (47)(47), 6330-6332 (2008-12-03)
A highly efficient and rapid total synthesis of 9Z-retinoic acid was accomplished by caesium fluoride-promoted Stille coupling reaction; using a common building block, 9Z-retinoic acid analogues were also prepared by the same method without isomerisation of the Z-double bond.
Nongmaithem Jiten Singh et al.
The journal of physical chemistry. B, 110(8), 3808-3815 (2006-02-24)
The structures, stabilities, thermodynamic quantities, dissociation energies, infrared spectra, and electronic properties of CsF hydrated by water molecules are investigated by using density functional theory, Møller-Plesset second-order perturbation theory (MP2), coupled cluster theory with singles, doubles, and perturbative triples excitations
Mitul K Patel et al.
Organic letters, 15(2), 346-349 (2013-01-05)
Phosphate esters in polyhydroxylated systems are normally blighted by uncontrolled migration under a variety of reaction conditions. Cesium fluoride is demonstrated as a reagent to control migration of primary phosphates during transesterifications. This allows easy exchange of phosphoryl protecting groups
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico