01580590
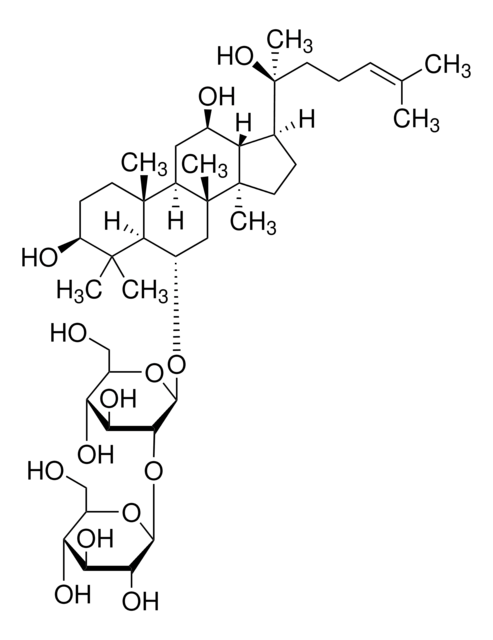
Ginsenoside Rf
primary reference standard
Sinónimos:
(3β,6α,12β)-3,12,20-Trihydroxydammar-24-en-6-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
grade
primary reference standard
shelf life
limited shelf life, expiry date on the label
manufacturer/tradename
HWI
application(s)
food and beverages
storage temp.
−20°C
SMILES string
[H][C@]1(O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@@]2([H])O[C@H]3C[C@]4(C)[C@]([H])(C[C@@H](O)[C@]5([H])[C@]([H])(CC[C@@]45C)[C@@](C)(O)CC\C=C(/C)C)[C@@]6(C)CC[C@H](O)C(C)(C)[C@]36[H]
InChI
1S/C42H72O14/c1-20(2)10-9-13-42(8,52)21-11-15-40(6)28(21)22(45)16-26-39(5)14-12-27(46)38(3,4)35(39)23(17-41(26,40)7)53-37-34(32(50)30(48)25(19-44)55-37)56-36-33(51)31(49)29(47)24(18-43)54-36/h10,21-37,43-52H,9,11-19H2,1-8H3/t21-,22+,23-,24+,25+,26+,27-,28-,29+,30+,31-,32-,33+,34+,35-,36-,37+,39+,40+,41+,42-/m0/s1
InChI key
UZIOUZHBUYLDHW-XUBRWZAZSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Exact content by quantitative NMR can be found on the certificate.
Application
Other Notes
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Optimize HPLC method for ginsenoside separation using a mixture, applying it to American Ginseng root, with conditions and chromatograms shown.
In this article we present several HPTLC applications and analytical standards for ginsenosides.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico