810603C
Avanti
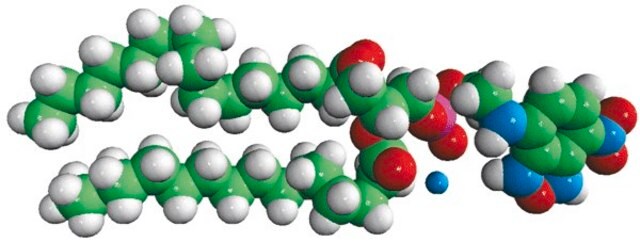
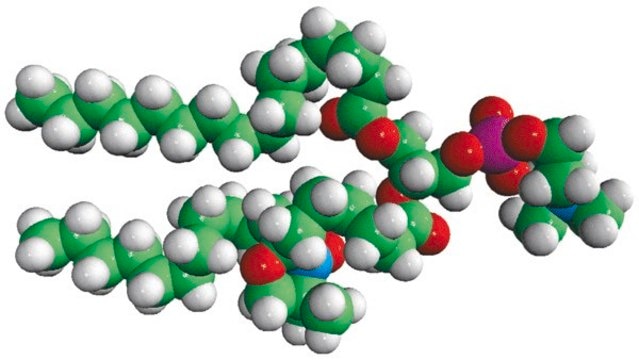
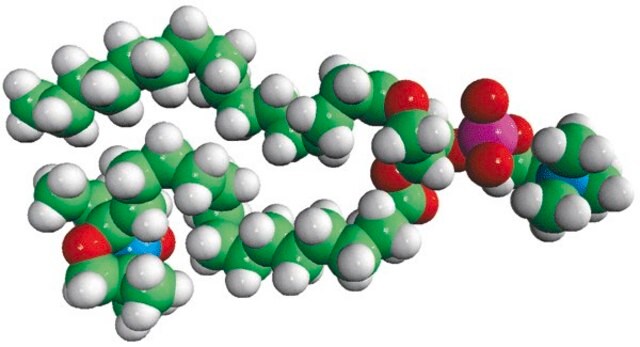
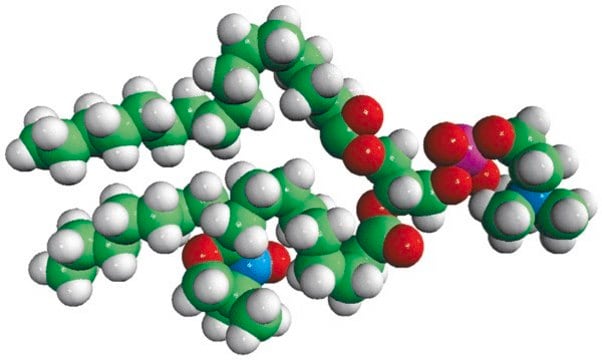
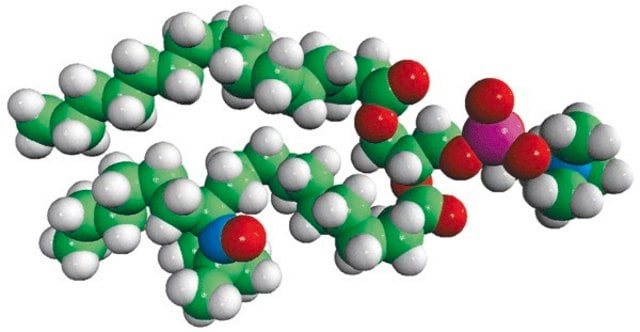
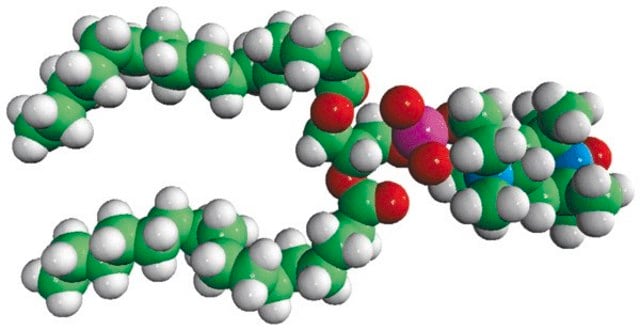
16:0-10 Doxyl PC
Avanti Research™ - A Croda Brand 810603C
Sinónimos:
1-palmitoyl-2-stearoyl-(10-doxyl)-sn-glycero-3-phosphocholine
About This Item
Productos recomendados
Ensayo
>99% (TLC)
Formulario
liquid
envase
pkg of 1 × 1 mL (810603C-1mg)
fabricante / nombre comercial
Avanti Research™ - A Croda Brand 810603C
concentración
1 mg/mL (810603C-1mg)
tipo de lípido
ESR probes
phospholipids
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
Descripción general
Aplicación
Acciones bioquímicas o fisiológicas
Envase
Nota de preparación
Información legal
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Carc. 2 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2 - STOT RE 1 - STOT SE 3
Órganos de actuación
Central nervous system, Liver,Kidney
Clase de riesgo para el agua (WGK)
WGK 3
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico