809160
N-Ethyl-N-isopropylpropan-2-aminium 3-(4-Acetyl-2,3,5,6-tetrafluorophenyl)-4-oxo-1,5-dioxaspiro[5.5]-undec-2-en-2-olate
About This Item
Productos recomendados
Formulario
powder
temp. de almacenamiento
2-8°C
cadena SMILES
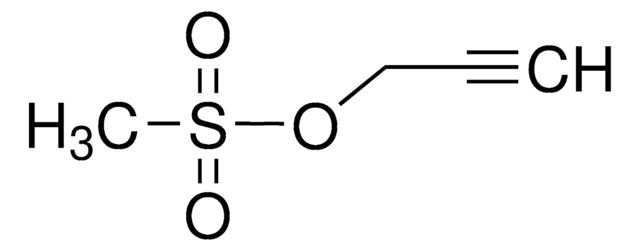
FC1=C(C(C)=O)C(F)=C(F)C(C2=C([O-])OC3(CCCCC3)OC2=O)=C1F.CC[NH+](C(C)C)C(C)C
InChI
1S/C17H14F4O5.C8H19N/c1-7(22)8-11(18)13(20)9(14(21)12(8)19)10-15(23)25-17(26-16(10)24)5-3-2-4-6-17;1-6-9(7(2)3)8(4)5/h23H,2-6H2,1H3;7-8H,6H2,1-5H3
Clave InChI
UYTIIFFVFGALQP-UHFFFAOYSA-N
Descripción general
Aplicación
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Contenido relacionado
Organofluorine chemistry is an essential part of drug discovery programs as well as agrochemical programs and even plays a major role in materials chemistry. Despite the undeniable importance of fluorinated organic molecules, our ability to synthesize these substrates is lacking - though arguably it is better than that of Nature. Consequently, methods that allow facile access to fluorinated molecules are important especially when they provide unique access to fluorinated chemical space.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico