344281
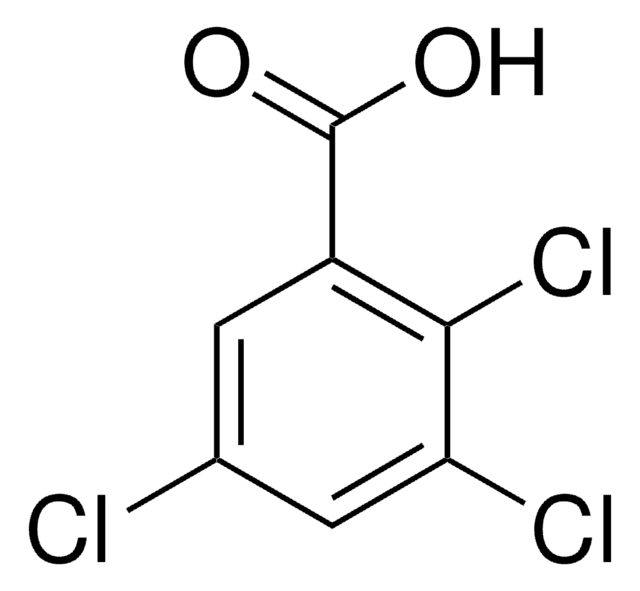
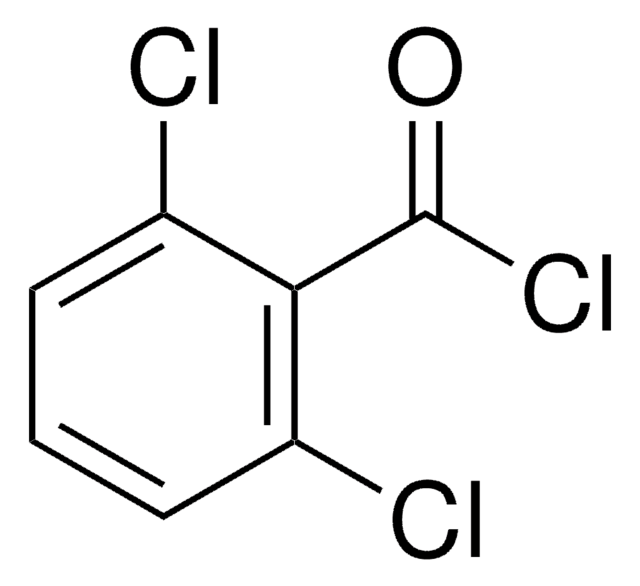
2,4,6-Trichlorobenzoic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Cl3C6H2CO2H
Número de CAS:
Peso molecular:
225.46
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
mp
160-164 °C (lit.)
grupo funcional
carboxylic acid
chloro
cadena SMILES
OC(=O)c1c(Cl)cc(Cl)cc1Cl
InChI
1S/C7H3Cl3O2/c8-3-1-4(9)6(7(11)12)5(10)2-3/h1-2H,(H,11,12)
Clave InChI
RAFFVQBMVYYTQS-UHFFFAOYSA-N
Descripción general
Structure and hydrogen bonding pattern in 2,4,6-trichlorobenzoic acid is reported.
Aplicación
2,4,6-Trichlorobenzoic acid may be employed as sole carbon and energy supplement for a microbial community. It may be used in the synthesis of (+)-methynolide, the aglycon of a macrolide antibiotic, methymycin.
Reactant involved in:
Cocatalyst for cis-dihydroxylation and epoxidation of alkenes
- Active-sodium-promoted reductive cleavage of halogenated benzoic acids
- Synthesis of aryl aminopyrazole benzamides for use as non-steroidal selective glucocorticoid receptor agonists
- Flame retardant monomer synthesis
- Synthesis of 3,4,7-trisubstituted coumarins for use as antifungals
- Solid-phase synthesis of saphenamycin analogs with antimicrobial activity
Cocatalyst for cis-dihydroxylation and epoxidation of alkenes
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
2, 4, 6-Trichlorobenzoic acid: Structure and hydrogen-bonding pattern.
Lalancette RA, et al.
Acta Crystallographica Section C, Structural Chemistry, 52(7), 1801-1804 (1996)
S Moller et al.
Applied and environmental microbiology, 63(6), 2432-2438 (1997-06-01)
A microbial community was cultivated in flow cells with 2,4,6-trichlorobenzoic acid (2,4,6-TCB) as sole carbon and energy source and was examined with scanning confocal laser microscopy and fluorescent molecular probes. The biofilm community which developed under these conditions exhibited a
F R Johannsen et al.
Journal of applied toxicology : JAT, 7(1), 67-70 (1987-02-01)
The acute rat oral LD50 of 2,4,6-Trichlorobenzyl chloride (TCBC) was determined to be 3075 mg/kg. When male and female rats were administered 1500 and 3000 ppm TCBC in the diet for 3 weeks, marked retardation in weight gain was observed.
Hong-Se Oh et al.
Organic & biomolecular chemistry, 7(21), 4458-4463 (2009-10-16)
Methynolide and 10-epi-methynolide were synthesized from the necessary segments, which were prepared by the addition of Grignard reagents to the corresponding alpha-alkoxyketones utilizing 1,2-stereochemical selection based on Cram chelation control. Ring-closing metathesis, as the key reaction, was carried out to
Kevin X Zhang et al.
Nature, 585(7825), 420-425 (2020-09-04)
The opsin family of G-protein-coupled receptors are used as light detectors in animals. Opsin 5 (also known as neuropsin or OPN5) is a highly conserved opsin that is sensitive to visible violet light1,2. In mice, OPN5 is a known photoreceptor
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico