128791
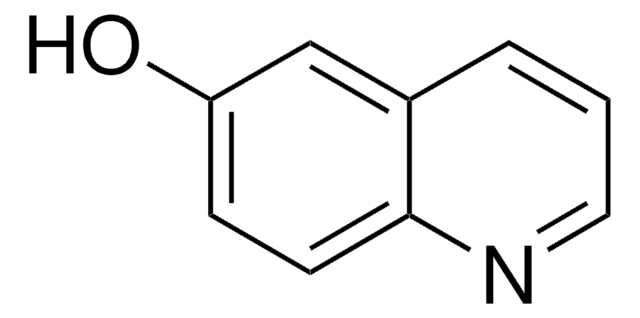
5-Quinolinol
99%
Sinónimos:
5-Hydroxyquinoline
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H7NO
Número de CAS:
Peso molecular:
145.16
Beilstein/REAXYS Number:
114514
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
99%
form
solid
mp
223-226 °C (lit.)
SMILES string
Oc1cccc2ncccc12
InChI
1S/C9H7NO/c11-9-5-1-4-8-7(9)3-2-6-10-8/h1-6,11H
InChI key
GYESAYHWISMZOK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
5-Quinolinol (5-Hydroxyquinoline) was used as an internal standard in the reaction to measure morphine concentration in serum or plasma. It was also used as a lipophilic chelator and it decreased the rate of deoxygenation.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Analysis of morphine in serum by high performance liquid chromatography with amperometric detection.
H Vandenberghe et al.
Therapeutic drug monitoring, 4(3), 307-314 (1982-01-01)
In this report we describe a rapid and sensitive micromethod using high performance liquid chromatography (HPLC) with electrochemical detection (ED) to measure morphine concentration in serum or plasma. The separation of morphine and the internal standard 5-hydroxyquinoline, from interfering compounds
M Hollstein et al.
Journal of the National Cancer Institute, 60(2), 405-410 (1978-02-01)
Quinoline, a hepatocarcinogen in rats, and 23 quinoline derivatives were tested for mutagenic activity with the Ames Salmonella typhimurium assay. Quinoline, 5-hydroxyquinoline, and 8-hydroxyquinoline were mutagenic in strain TA 100 when Aroclor 1254-induced rat (male outbred Sprague-Dawley) liver homogenate was
R Deslauriers et al.
Biochimica et biophysica acta, 886(3), 319-326 (1986-05-29)
A spectrophotometric assay has been devised to measure oxygen consumption non-invasively in intact murine red cells parasitized by Plasmodium berghei. The method uses oxyhemoglobin in the erythrocytes both as a source of oxygen and as an indicator of oxygen consumption.
Markus Brinkmann et al.
Chemical research in toxicology, 32(4), 698-707 (2019-03-22)
Hydroxylation of polyaromatic compounds through cytochromes P450 (CYPs) is known to result in potentially estrogenic transformation products. Recently, there has been an increasing awareness of the importance of alternative pathways such as aldehyde oxidases (AOX) or N-methyltransferases (NMT) in bioactivation
Nihal Kuş et al.
The journal of physical chemistry. A, 119(24), 6296-6308 (2015-05-31)
The structure, infrared spectrum, and photochemistry of 5-hydroxyquinoline (5HQ) were studied by matrix isolation infrared spectroscopy, complemented by theoretical calculations performed at the DFT(B3LYP)/6-311++G(d,p) level of approximation. According to the calculations, the trans conformer of 5HQ (with the OH group
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico