General description
We are committed to bringing you greener alternative products, which adhere to one or more of The 12 Principles of Green Chemistry.This antibody is Preservative-free, produced without the harm or sacrifice of animals and exceptionally stable to allow for ambient shipping and storage if needed and thus aligns with "Waste Prevention", "Designing Safer Chemicals" and "Design for Energy Efficiency".
Click here for more information.
ZooMAb® antibodies represent an entirely new generation of recombinant monoclonal antibodies.Each ZooMAb® antibody is manufactured using our proprietary recombinant expression system, purified to homogeneity, and precisely dispensed to produce robust and highly reproducible lot-to-lot consistency. Only top-performing clones are released for use by researchers. Each antibody is validated for high specificity and affinity across multiple applications, including its most commonly used application. ZooMAb® antibodies are reliably available and ready to ship when you need them.
Specificity
Clone 1C18 is a ZooMAb® Rabbit recombinant monoclonal antibody that specifically detects HLA class I histocompatibility antigen, alpha chain G (HLA-G). It targets an epitope within 18 amino acids from the extracellular domain within the N-terminal half.
Immunogen
KLH-conjugated linear peptide corresponding to 18 amino acids from the extracellular domain within the N-terminal half of human HLA class I histocompatibility antigen, alpha chain G (HLA-G).
Application
Quality Control Testing
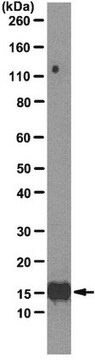
Evaluated by Western Blotting in Jurkat cell lysate.
Western Blotting Analysis: A 1:1,000 dilution of this antibody detected HLA-G in Jurkat cell lysate.
Tested applications
Affinity Binding Assay: A representative lot of this antibody bound HLA-G peptide with a KD of 2.4 x 10-7 in an affinity binding assay.
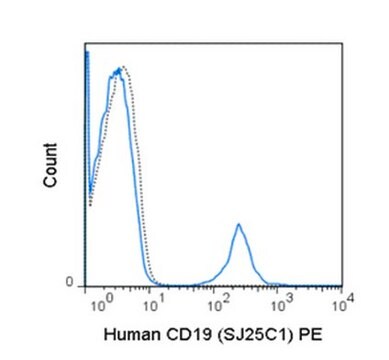
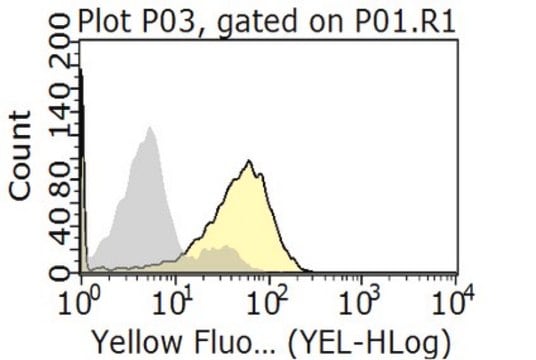
Flow Cytometry Analysis: 1 μg from a representative lot detected HLA-G in one million JEG3 cells.
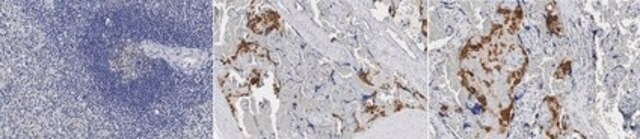
Immunohistochemistry (Paraffin) Analysis: A 1:1,000 dilution from a representative lot detected HLA-G in human placenta tissue sections.
Note: Actual optimal working dilutions must be determined by end user as specimens, and experimental conditions may vary with the end user
Evaluated by Western Blotting in Jurkat cell lysate.
Western Blotting Analysis: A 1:1,000 dilution of this antibody detected HLA-G in Jurkat cell lysate.
Target description
HLA class I histocompatibility antigen, alpha chain G (UniProt: P17693; also known as HLA G antigen, MHC class I antigen G) is encoded by the HLA-G (also known as HLA-6.0, HLAG) gene (Gene ID: 3135) in human. HLA-G is a class I histocompatibility antigen, but unlike the classical class I major histocompatibility complex, it displays limited polymorphism. Seven different isoforms of HLA-G have been described that are produced by alternative splicing. With the exception of isoform 1, other isoforms lack a portion of their extracellular domain. HLA-G is a single-pass type I membrane protein that is synthesized with a signal peptide (aa 1-24), which is subsequently cleaved off to generate the mature form that contains an extracellular domain (aa 25-308), a transmembrane domain (aa 309-332), and a short cytoplasmic tail (aa 333-338). It is also present on endoplasmic reticulum and endosomes. HLA-G expression is observed in adult eye and in immune cell subsets including monocytes, myeloid and plasmacytoid dendritic cells, and regulatory T cells. It is also expressed abundantly and specifically on extravillous trophoblasts (EVT) in physiological conditions. Soluble HLA-G is also found in maternal blood, amniotic fluid, and cord blood and participates in maternal-fetal immune tolerance by keeping in check the effector functions of NK, CD8+ T cells, and B cells. Its expression is detected as early as the morula stage. HLA-G gene polymorphisms and reduced levels of soluble HLA-G have been linked to failure of embryo implantation, recurrent spontaneous abortions, and placental abruption. HLA-G is considered as a critical marker of immunotolerance in cancer cell immune evasion and is strongly associated with disease progress and prognosis for cancer patients. Its expression and isoform profile varies significantly among tumor types and between primary tumor and metastases. This ZooMAbZooMAb® recombinant monoclonal antibody, generated by our propriety technology, offers significantly enhanced specificity, affinity, reproducibility, and stability over conventional monoclonals. (Ref.: Xu, X., et al. (2020). Front. Immunol. 11; 592010; Lin, A., and Yan, W-H. (2018). Front. Immunol. 9; 2164).
Physical form
Purified recombinant rabbit monoclonal antibody IgG, lyophilized in PBS, 5% Trehalose, normal appearance a coarse or translucent resin. The PBS/trehalose components in the ZooMAb formulation can have the appearance of a semi-solid (bead like gel) after lyophilization. This is a normal phenomenon. Please follow the recommended reconstitution procedure in the data sheet to dissolve the semi-solid, bead-like, gel-appearing material. The resulting antibody solution is completely stable and functional as proven by full functional testing. Contains no biocide or preservatives, such as azide, or any animal by-products. Larger pack sizes provided as multiples of 25 μL.
Reconstitution
300 μg/mL after reconstitution at 25 μL per vial. Please refer to guidance on suggested starting dilutions and/or titers per application and sample type.
Storage and Stability
Recommend storage of lyophilized product at 2-8°C; Before reconstitution, micro-centrifuge vials briefly to spin down material to bottom of the vial; Reconstitute each vial by adding 25 μL of filtered lab grade water or PBS; Reconstituted antibodies can be stored at 2-8°C, or -20°C for long term storage. Avoid repeated freeze-thaws.
Legal Information
ZooMAb is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.