C6271
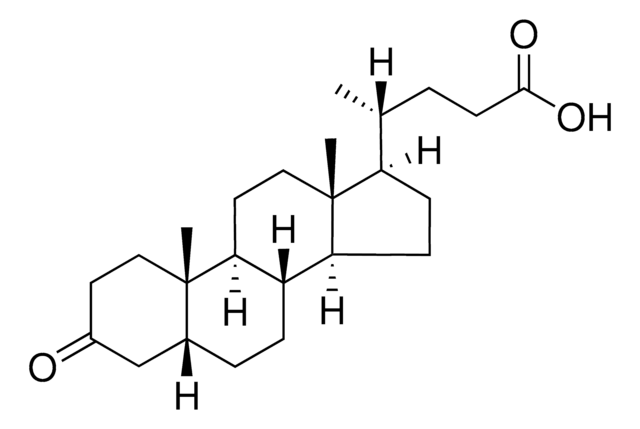
5β-Cholanic acid-3-one
Synonym(s):
3-Keto-5β-cholanic acid, Dehydrolithocholic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C24H38O3
CAS Number:
Molecular Weight:
374.56
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
SMILES string
[H][C@]12CC[C@@]3([H])[C@]4([H])CC[C@]([H])([C@H](C)CCC(O)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CCC(=O)C2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y Amuro et al.
Biochimica et biophysica acta, 837(1), 20-26 (1985-10-23)
The formation of lithocholic and isolithocholic acids from 3-keto-5 beta-cholanoic acid by human liver cytosol was examined in vitro. Liver cytosol was incubated at various pH levels with 3-keto-5 beta-cholanoic acid in a phosphate buffer containing NADPH or NADH; the
R W Owen et al.
Journal of steroid biochemistry, 22(6), 817-822 (1985-06-01)
The metabolism of unsaturated bile acids and androstanes by mixed human faecal cultures has been studied. The reactions observed were mainly reductive. Unsaturated 4-ene-3-oxo and 1,4-diene-3-oxo bile acids were reduced in Ring A. 5 beta-3-Oxo bile acids were reduced to
Hiroyuki Sato et al.
Journal of medicinal chemistry, 51(6), 1831-1841 (2008-03-01)
TGR5, a metabotropic receptor that is G-protein-coupled to the induction of adenylate cyclase, has been recognized as the molecular link connecting bile acids to the control of energy and glucose homeostasis. With the aim of disclosing novel selective modulators of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service