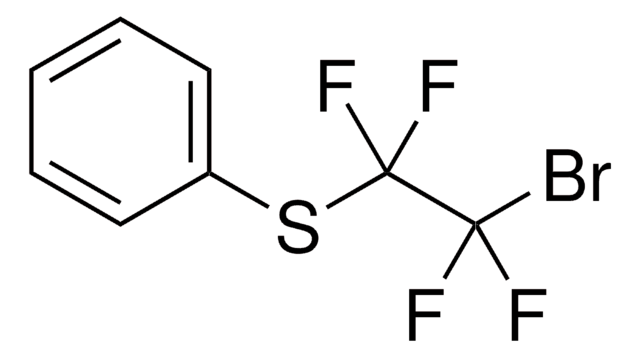
Recommended Products
form
liquid
reaction suitability
reaction type: C-C Bond Formation
Application
Automate your fluorination reactions with Synple Automated Synthesis Platform (SYNPLE-SC002)
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Fluoroalkylation toolbox expands with various reagents for late-stage fluoroalkylation in organic synthesis and medicinal chemistry.
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

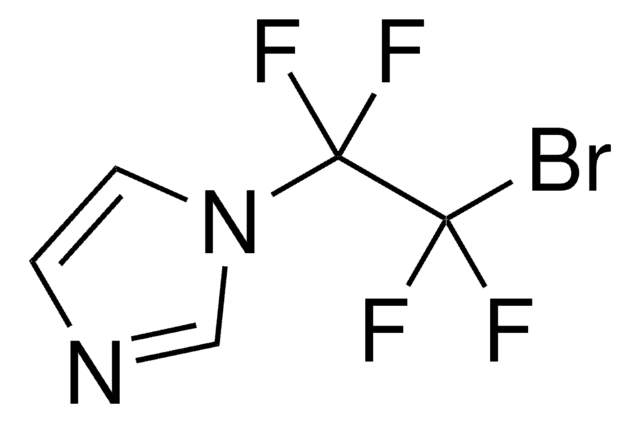
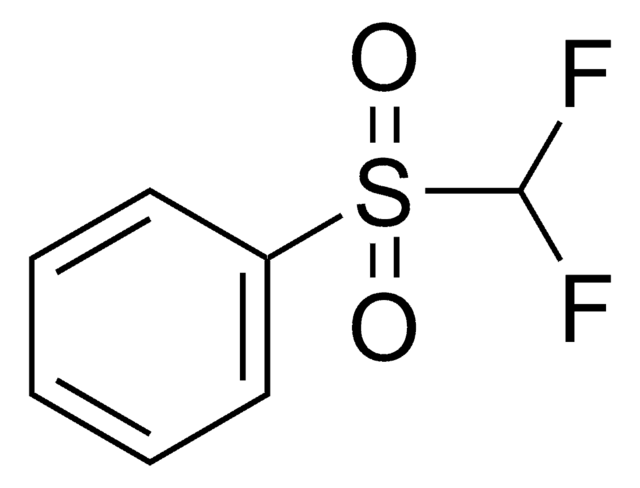
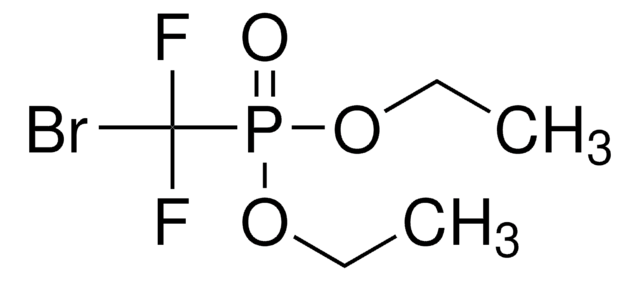

![Diethyl [difluoro(trimethylsilyl)methyl]phosphonate ≥85.0% (H-NMR)](/deepweb/assets/sigmaaldrich/product/structures/882/287/1ae5ac59-32da-4395-8401-85d66ff7f69f/640/1ae5ac59-32da-4395-8401-85d66ff7f69f.png)