794236
PTAD-Alkyne
97%
Synonym(s):
4-(4-(Prop-2-yn-1-yloxy)phenyl)-1,2,4-triazolidine-3,5-dione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H9N3O3
CAS Number:
Molecular Weight:
231.21
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
176-181 °C
storage temp.
2-8°C
SMILES string
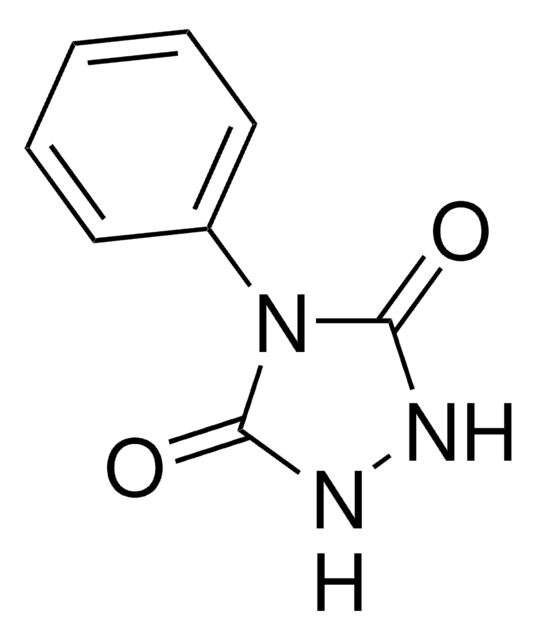
O=C1NNC(=O)N1c2ccc(OCC#C)cc2
InChI
1S/C11H9N3O3/c1-2-7-17-9-5-3-8(4-6-9)14-10(15)12-13-11(14)16/h1,3-6H,7H2,(H,12,15)(H,13,16)
InChI key
IHJYYZNVZZKSCU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
PTAD-Alkyne is a selective bioconjugation reagent for the modification of tyrosine residues with a terminal alkyne. Subsequent modification can then be afforded via the copper catalyzed azide/alkyne click chemistry reaction with an azide containing compound or biomolecule. This reagent has been shown to selectively introduce poly(ethylene glycol) or PEG chains onto proteins with surface exposed tyrosine residues. PTAD-Alkyne has also been used in the formation of antibody-drug conjugates. This reagent is compatible with different buffer systems such as PBS, Tris and mixed PBS/Tris buffer (preferred). The linkage with tyrosine has been shown to be stable to pH and temperature extremes as well as blood plasma.
Application
Note: PTAD-Alkyne must be first activated by stirring in a 1:0.98 molar ratio with 1,3-dibromo-5,5-dimethylhydantoin (product # 157902). Activation is evident upon solution color change from colorless to deep red and the activated reagent should be used immediately.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile; avoid using DMSO)
Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
Remove excess unreacted PTAD by gel filtration.
General Procedure for Protein Modification with PTAD.
Part 1: PTAD activation
Mix together 1:0.98 molar equivalents of unactivated PTAD to 1,3-dibromo-5,5-dimethylhydantoin (product # 157902) in organic solvent (preferred solvents are DMF or acetonitrile; avoid using DMSO)
Color change is observed from colorless/pale yellow to deep red (approximately 5 min of mixing).
After the solution turns red, store the now activated reagent on ice and use for protein modification within 30 min.
Part 2: Protein modification
Add protein solution in mixed phosphate/Tris buffer or Tris buffer (pH should be 6 - 9) to the eppendorf tube (or other vial) containing the activated PTAD reagent prepared above and mix gently at room temperature for up to 30 min. Preferably use 10-fold molar excess of reagent relative to protein. Use protein at a minimum concentration of 1 mg/ml (higher concentrations are preferred for enhanced labeling).
Remove excess unreacted PTAD by gel filtration.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hitoshi Ban et al.
Bioconjugate chemistry, 24(4), 520-532 (2013-03-29)
The scope, chemoselectivity, and utility of the click-like tyrosine labeling reaction with 4-phenyl-3H-1,2,4-triazoline-3,5(4H)-diones (PTADs) is reported. To study the utility and chemoselectivity of PTAD derivatives in peptide and protein chemistry, we synthesized PTAD derivatives possessing azide, alkyne, and ketone groups
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service