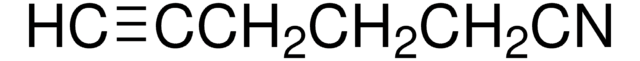All Photos(1)
About This Item
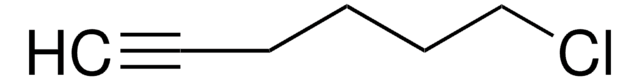
Linear Formula:
CH2=CHCH2CH2CN
CAS Number:
Molecular Weight:
81.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.42 (lit.)
bp
140 °C (lit.)
density
0.814 g/mL at 25 °C (lit.)
SMILES string
C=CCCC#N
InChI
1S/C5H7N/c1-2-3-4-5-6/h2H,1,3-4H2
InChI key
CFEYBLWMNFZOPB-UHFFFAOYSA-N
General description
4-Pentenenitrile (4-PN) is a terminal alkene nitrile. It is obtained from 3-pentenenitrile via cationic nickel hydride or cobalt catalyzed isomerization. 4-Pentenenitrile undergoes hydrocyanation in the presence of bidentate nickel complexes (catalysts) to yield 3-pentenenitrile. 4-PN undergoes gas-phase reaction with OH radicals and Cl atoms in the presence of synthetic air and various reference compounds.
Application
4-Pentenenitrile may be used to synthesize 4-pentenylamine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
B Ozierenski et al.
Die Nahrung, 37(1), 5-14 (1993-01-01)
The effects of 5-vinyloxazolidine-2-thione (VOT), 1-cyano-3-butene (CYB) and various isothiocyanates on parameters of hepatic phase I and phase II biotransformation were investigated in male rats after oral treatment for 3 consecutive days. The compounds with the exception of CYB caused
Ligand Descriptor Analysis in Nickel-Catalysed Hydrocyanation: A Combined Experimental and Theoretical Study.
Burello E, et al.
Advanced Synthesis & Catalysis, 347(6), 803-810 (2005)
Gas-phase reactivity study of (E)-3-pentenenitrile and 4-pentenenitrile towards OH radicals and Cl atoms at atmospheric pressure.
Colomer JP, et al.
Atmospheric Environment, 61, 597-604 (2012)
New methods for alkaloid synthesis: Generation of indole-2, 3-diquinomethanes as a route to indole alkaloids.
Gallagher T and Magnus P.
Tetrahedron, 37(23), 3889-3897 (1981)
Kinetic control in catalytic olefin isomerization. An explanation for the apparent contrathermodynamic isomerization of 3-pentenenitrile.
McKinney RJ.
Organometallics, 4(6), 1142-1143 (1985)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service