480061
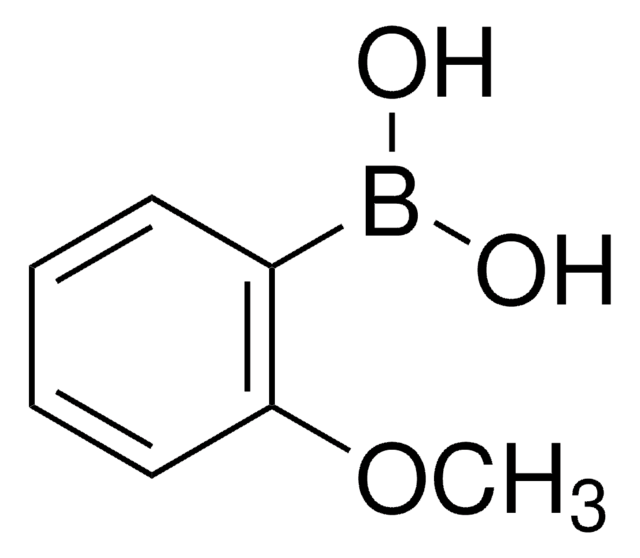
2,6-Dimethylphenylboronic acid
≥95.0%
Synonym(s):
2,6-Dimethylbenzeneboronic acid, 2,6-Xyleneboronic acid, 2,6-Xylylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C6H3B(OH)2
CAS Number:
Molecular Weight:
149.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0%
impurities
<10% water
mp
105 °C (dec.) (lit.)
SMILES string
Cc1cccc(C)c1B(O)O
InChI
1S/C8H11BO2/c1-6-4-3-5-7(2)8(6)9(10)11/h3-5,10-11H,1-2H3
InChI key
ZXDTWWZIHJEZOG-UHFFFAOYSA-N
Related Categories
Application
Reagent used for
Reagent used in Prepration of
- Palladium catalyzed Suzuki-Miyaura coupling reactions
- One-pot ipso-nitration of arylboronic acids including broader substrate scope of heterocycles and functional groups
- Nickel-Catalyzed Cross-Coupling of Chromene Acetals and Boronic Acids
- Visible-light initiated aerobic oxidative hydroxylation catalyzed by Ru-complex
- Rhodium(I)-catalyzed 1,4-addition reactions
- Pd-catalyzed homocouplings
- Expanded scope of Cu assisted Suzuki-Miyaura coupling reactions including aryl chlorides and polyhalo aryl boronates
Reagent used in Prepration of
- Orally bioavialable G Protein-Coupled Receptor 40 agonists for diabetes treatment
- Solid phase synthesis and antitumor structure-activity relationship of Smac triazoloprolines and biarylalanines tetrapeptide libraries
- Protein Kinase inhibitors
Other Notes
contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Santos-Filho, E. F.; et al.
Tetrahedron Letters, 52, 5288-5288 (2011)
Rhodium(I)-catalyzed 1,4-addition of arylboronic acids to acrylic acid in water: one-step preparation of 3-arylpropionic acids
Vautravers, N. R.; Breit, B.
Synlett, 17, 2517-2520 (2011)
Satoshi Mikami et al.
Journal of medicinal chemistry, 55(8), 3756-3776 (2012-03-21)
As part of a program to identify potent GPR40 agonists with drug-like properties suitable for clinical development, the incorporation of polar substituents was explored with the intention of decreasing the lipophilicity of our recently disclosed phenylpropanoic acid derivative 1. This
Thomas J A Graham et al.
Organic letters, 14(6), 1616-1619 (2012-03-06)
A modular and highly efficient protocol for the synthesis of 2-aryl- and heteroaryl-2H-chromenes is described. Under base-free conditions, readily accessible 2-ethoxy-2H-chromenes undergo C(sp(3))-O activation and C(sp(3))-C bond formation in the presence of an inexpensive nickel catalyst and boronic acids. This
Room-temperature synthesis of tetra-ortho-substituted biaryls by NHC-catalyzed Suzuki-Miyaura couplings.
Linglin Wu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(46), 12886-12890 (2011-10-11)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service