All Photos(1)
About This Item
Empirical Formula (Hill Notation):
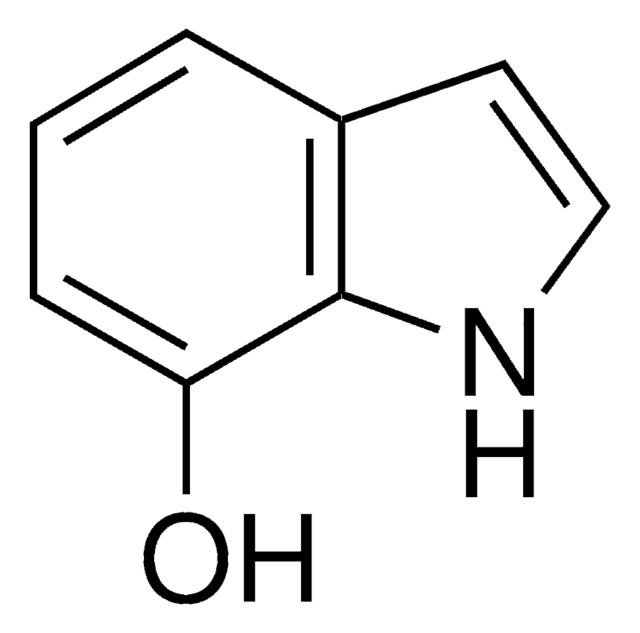
C10H11NO2
CAS Number:
Molecular Weight:
177.20
Beilstein:
5683
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
154-157 °C (lit.)
SMILES string
COc1cc2cc[nH]c2cc1OC
InChI
1S/C10H11NO2/c1-12-9-5-7-3-4-11-8(7)6-10(9)13-2/h3-6,11H,1-2H3
InChI key
QODBZRNBPUPLEZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5,6-Dimethoxyindole was used in the synthesis of N-benzyl-N-cyclopropyl-5,6-dimethoxyindole-3-glyoxalamide.
- Reactant in synthesis of indolylhydroxyoxindoles via enantioselective Friedel-Crafts reaction
- Reactant in synthesis of benzyl trimethoxyindoles
- Reactant for synthesis of benzoylpiperazinyl-indolyl ethane dione derivatives as HIV-1 inhibitors
- Reactant for synthesis of 1-aroylindole 3-aroylindoles combretastatin A-4 analogs as antitumor agents and tubulin polymerization inhibitors
- Reactant for preparation of tryptophanol derivatives via the Grignard reaction
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W B Emary et al.
Rapid communications in mass spectrometry : RCM, 3(12), 413-416 (1989-12-01)
It is not possible to distinguish isomers of biologically important dimethoxyindoles using electron-ionization mass spectra, but they may be distinguished by collisionally activated dissociation. In particular, energy-resolved mass spectrometry yields the best data for distinguishing between these isomers.
J Hirschinger et al.
Solid state nuclear magnetic resonance, 3(3), 121-135 (1994-06-01)
The inversion-recovery cross-polarization (IRCP) magic-angle spinning experiment has been applied to study the 13C-1H cross-polarization dynamics of protonated aromatic carbons in ferrocene, 5,6-dimethoxyindole (DMI) and some indole derivatives. Using the 13C-detected proton spin diffusion (SD) experiment recently developed by Zhang
F Chimenti et al.
Il Farmaco; edizione scientifica, 35(9), 785-790 (1980-09-01)
The synthesis of two new N-cyclopropyltryptamines is described. By treating 5,6-dimethoxyindole with oxalyl chloride and N-benzylcyclopropylamine, N-benzyl-N-cyclopropyl-5,6-dimethoxyindole-3-glyoxalamide is obtained. The reduction of this compound by LiAlH4, gives N-benzyl-N-cyclopropyl-5,6-dimethoxytryptamine, which is hydrogenated to N-cyclopropyl-5,6-dimethoxytryptamine. Similarly N-cyclopropyl-6,7-dimethoxytryptamine is prepared. Preliminary results indicate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service