180084
2,2′-Sulfonyldiethanol solution
60-65 wt. % in H2O
Synonym(s):
2-Hydroxyethyl sulfone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
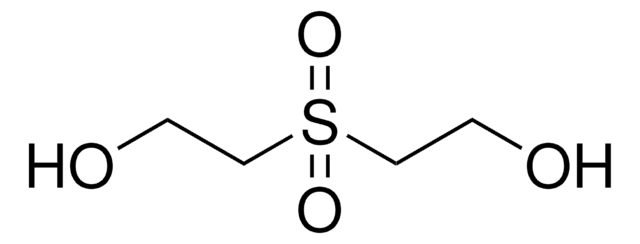
O2S(CH2CH2OH)2
CAS Number:
Molecular Weight:
154.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
5.5 (vs air)
vapor pressure
23.5 mmHg ( 20 °C)
concentration
60-65 wt. % in H2O
density
1.236 g/mL at 25 °C
SMILES string
OCCS(=O)(=O)CCO
InChI
1S/C4H10O4S/c5-1-3-9(7,8)4-2-6/h5-6H,1-4H2
InChI key
QQLILYBIARWEIF-UHFFFAOYSA-N
Application
2,2′-Sulfonyldiethanol was used as a cross-linking agent for poly(vinyl phosphonic acid) (PVPA).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R M Black et al.
Journal of chromatography. B, Biomedical applications, 665(1), 97-105 (1995-03-10)
Gas chromatography-tandem mass spectrometry (GC-MS-MS) with selected-reaction monitoring was applied to the analysis of urinary metabolites of sulphur mustard, derived from the beta-lyase pathway and from hydrolysis. In the case of beta-lyase metabolites, a limit of detection of 0.1 ng/ml
R M Black et al.
Xenobiotica; the fate of foreign compounds in biological systems, 25(2), 167-173 (1995-02-01)
1. Samples of urine from two human subjects accidentally exposed to sulphur mustard were analysed for metabolites derived from hydrolysis (thiodiglycol, thiodiglycol sulphoxide), conjugation with glutathione (1,1'-sulphonylbis [2-S-(N-acetylcysteinyl)ethane]) and from further metabolism of glutathione conjugates by the beta-lyase pathway (1,1-sulphonylbis[2-(methylsulphinyl)ethane]
R M Black et al.
Xenobiotica; the fate of foreign compounds in biological systems, 22(4), 405-418 (1992-04-01)
1. The metabolism of sulphur mustard, 1,1'-thiobis(2-chloroethane), in vivo was investigated following i.p. administration to rat. 2. Approx. 60% of dose was excreted in the 24 h urine. Many metabolites were present; nine have been isolated by h.p.l.c. and characterized
Richard T Pon et al.
Nucleic acids research, 32(2), 623-631 (2004-01-31)
New linker phosphoramidite reagents containing a cleavable 3'-ester linkage are used for attaching the first nucleoside to the surface of a solid- phase support. Inexpensive, underivatized amino supports, such as long chain alkylamine controlled-pore glass, can serve as universal supports.
R M Black et al.
Journal of chromatography, 558(2), 393-404 (1991-10-11)
Two methods have been developed for the analysis of thiodiglycol sulphoxide, a metabolite of sulphur mustard, in urine. The first method recovers thiodiglycol sulphoxide from urine by extraction from a solid absorbent tube and clean up on Florisil. In the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service