H4291
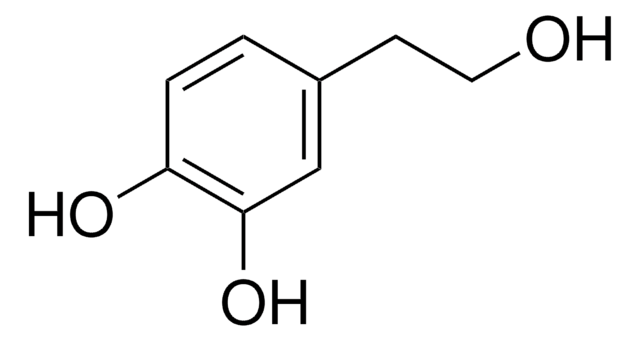
3-Hydroxytyrosol
≥98% (HPLC)
Synonym(s):
2-(3,4-Dihydroxyphenyl)ethanol, 3,4-Dihydroxyphenethyl alcohol, DOPET, Homoprotocatechuyl alcohol
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
storage temp.
−20°C
SMILES string
OCCc1ccc(O)c(O)c1
InChI
1S/C8H10O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,9-11H,3-4H2
InChI key
JUUBCHWRXWPFFH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Production of 3-Hydroxytyrosol from Glucose by Chromosomally Engineered Escherichia coli by Fed-Batch Cultivation in a Jar Fermenter.: Demonstrates biotechnological production of 3-Hydroxytyrosol using genetically modified E. coli, highlighting scalable methods for synthesizing valuable biochemicals from simple sugars (Koma et al., 2023).
- Green Extraction of Antioxidant Compounds from Olive Tree Leaves Based on Natural Deep Eutectic Solvents.: Investigates eco-friendly extraction methods for recovering 3-Hydroxytyrosol from olive leaves, emphasizing sustainable chemical processes and the high antioxidant potential of the extracts (Mir-Cerdà et al., 2023).
Biochem/physiol Actions
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service