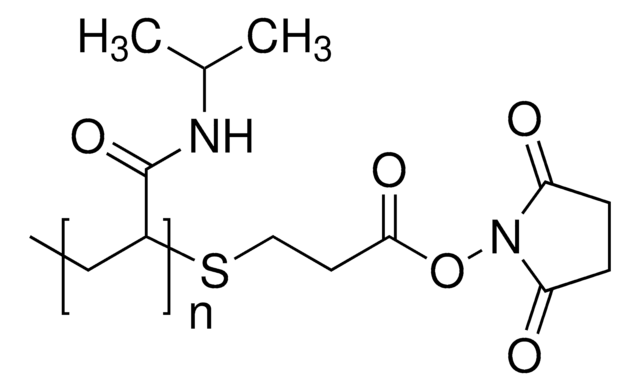
56480
N-Hydroxysuccinimide
≥97.0% (T), for peptide synthesis
Synonym(s):
1-Hydroxy-2,5-pyrrolidinedione, HOSu, NHS
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C4H5NO3
CAS Number:
Molecular Weight:
115.09
Beilstein:
113913
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
N-Hydroxysuccinimide, purum, ≥97.0% (T)
grade
purum
Quality Level
Assay
≥97.0% (T)
form
solid
reaction suitability
reaction type: Addition Reactions
mp
95-98 °C (lit.)
95-98 °C
application(s)
peptide synthesis
functional group
imide
SMILES string
ON1C(=O)CCC1=O
InChI
1S/C4H5NO3/c6-3-1-2-4(7)5(3)8/h8H,1-2H2
InChI key
NQTADLQHYWFPDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-Hydroxysuccinimide (NHS) can be used in the following applications:
- To synthesize N-succinimidyl 3-(2-pyridyldithio)-propionate, a heterobifunctional reagent useful for protein-protein conjugation and also to incorporate aliphatic thiols into proteins.
- To synthesize NHS esters of long-chain fatty acids.
- NHS can activate the phosphonic acid monolayers immobilized on titanium surface for binding with proteins.
Other Notes
may contain 1-3% succinic acid and/or succinic anhydride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio) propionate, a new heterobifunctional reagent.
Carlsson J
The Biochemical Journal, 173(3), 723-723 (1978)
Synthesis of long-chain fatty acyl-CoA thioesters using N-hydroxysuccinimide esters.
Blecher M
Methods in Enzymology, 72, 404-408 (1981)
Phosphonic acid monolayers for binding of bioactive molecules to titanium surfaces.
Adden N
Langmuir, 22(19), 8197-8204 (2006)
Burcu Ayoglu et al.
EMBO molecular medicine, 6(7), 918-936 (2014-06-13)
Despite the recent progress in the broad-scaled analysis of proteins in body fluids, there is still a lack in protein profiling approaches for biomarkers of rare diseases. Scarcity of samples is the main obstacle hindering attempts to apply discovery driven
Kengo Nishi et al.
The Journal of chemical physics, 137(22), 224903-224903 (2012-12-20)
We investigated the relationship between the elastic modulus, G and the reaction probability, p for polymer networks. First, we pointed out that the elastic modulus is expressed by G = {(fp∕2 - 1) + O((p - 1)(2))} Nk(B)T∕V (percolated network
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service