W313009
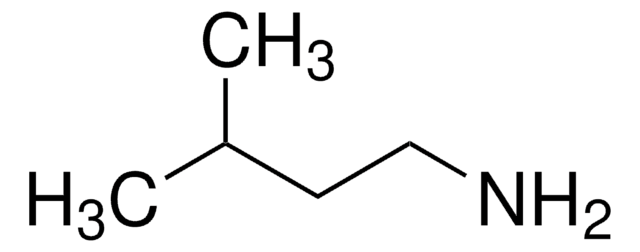
Butylamine
≥99%
Synonym(s):
1-Aminobutane, n-Butylamine
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Halal
Kosher
Agency
meets purity specifications of JECFA
vapor density
2.5 (vs air)
vapor pressure
68 mmHg ( 20 °C)
Assay
≥99%
autoignition temp.
594 °F
expl. lim.
9.8 %
refractive index
n20/D 1.401 (lit.)
bp
78 °C (lit.)
mp
−49 °C (lit.)
density
0.74 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
fishy; meaty
SMILES string
CCCCN
InChI
1S/C4H11N/c1-2-3-4-5/h2-5H2,1H3
InChI key
HQABUPZFAYXKJW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Characteristics of Purified Horse Oil by Supercritical Fluid Extraction with Different Deodorants Agents.: This study explores the purification of horse oil using supercritical fluid extraction, focusing on the effectiveness of various deodorant agents including butylamine. The research demonstrates the potential of butylamine in enhancing the quality and usability of horse oil in food and pharmaceutical applications (Anneke et al., 2024).
- A divergent one-pot thiol-Michael strategy to create β-thiophene-fused porphyrins.: This paper presents a novel one-pot thiol-Michael addition strategy to synthesize β-thiophene-fused porphyrins. The use of butylamine in the reaction demonstrates its utility in organic synthesis and potential applications in developing new materials for electronic and photonic devices (Singh et al., 2024).
- Strain engineering improves the photovoltaic performance of carbon-based hole-transport-material free CsPbIBr(2) perovskite solar cells.: This study enhances the performance of perovskite solar cells through strain engineering. Butylamine is utilized in the process, showcasing its application in improving the efficiency of renewable energy technologies (He et al., 2024).
Disclaimer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Met. Corr. 1 - Skin Corr. 1A - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service