W309311
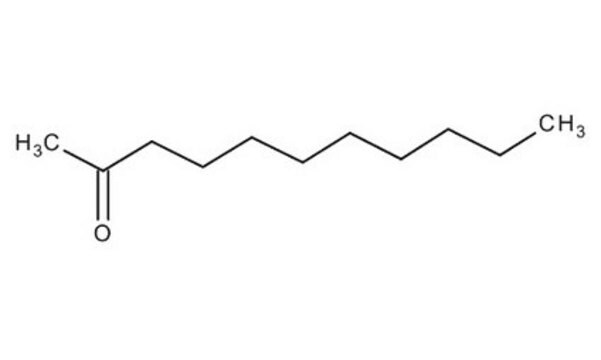
2-Undecanone
natural, FCC, FG
Synonym(s):
Methyl nonyl ketone
About This Item
Recommended Products
grade
FG
Halal
Kosher
natural
Quality Level
Agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
vapor density
5.9 (vs air)
vapor pressure
<1 mmHg ( 20 °C)
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.43 (lit.)
bp
231-232 °C (lit.)
mp
11-13 °C (lit.)
density
0.825 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
greener alternative category
Organoleptic
fatty; waxy; fruity
SMILES string
CCCCCCCCCC(C)=O
InChI
1S/C11H22O/c1-3-4-5-6-7-8-9-10-11(2)12/h3-10H2,1-2H3
InChI key
KYWIYKKSMDLRDC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Larvicididal Activity of Natural Repellents Against the Dengue Vector, Aedes aegypti: This paper details the use of natural compounds like 2-Undecanone as effective larvicidal agents against vectors of significant human diseases, showcasing their role in developing non-toxic, eco-friendly insect control solutions (Zhang et al., 2020).
- Control of Filth Flies, Cochliomyia macellaria (Diptera: Calliphoridae), Musca domestica (Diptera: Muscidae), and Sarcophaga bullata (Diptera: Sarcophagidae), Using Novel Plant-Derived Methyl Ketones: Investigates the efficacy of 2-Undecanone in managing common pest flies, underlining its potential as a biopesticide and organic solvent in public health and safety applications (Deguenon et al., 2019).
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
192.2 °F - closed cup
Flash Point(C)
89 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service