N1607
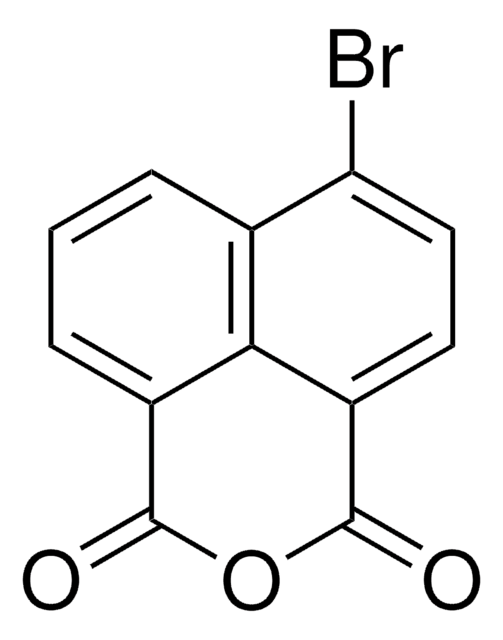
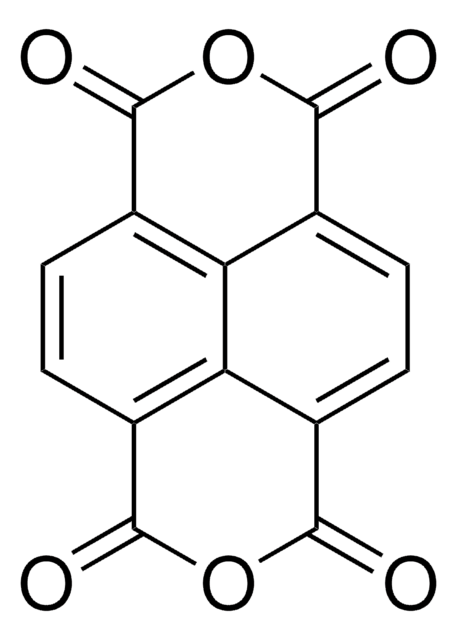
1,8-Naphthalic anhydride
Synonym(s):
Naphtho[1,8,8a-c,d]pyran-1,3-dione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H6O3
CAS Number:
Molecular Weight:
198.17
Beilstein:
153190
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
mp
267-269 °C (lit.)
SMILES string
O=C1OC(=O)c2cccc3cccc1c23
InChI
1S/C12H6O3/c13-11-8-5-1-3-7-4-2-6-9(10(7)8)12(14)15-11/h1-6H
InChI key
GRSMWKLPSNHDHA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
521.6 °F
Flash Point(C)
272 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lijuan Xie et al.
Bioorganic & medicinal chemistry, 17(2), 804-810 (2008-12-17)
A series of 5-alkylamino substituted amonafide analogues were synthesized from naphthalic anhydride by three steps including bromization, amination and CuI/proline catalyzed coupling reaction. The CuI/L-proline catalyzed coupling reaction was first applied to the naphthalimide system. These new amonafide analogues showed
Nikolai I Georgiev et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 183, 7-16 (2017-04-23)
Two novel highly water-soluble fluorescence sensing 1,8-naphthalimides are synthesized and investigated. The novel compounds are designed on the "fluorophore-receptor
J Sauther et al.
The Journal of chemical physics, 131(3), 034711-034711 (2009-07-25)
The pi-conjugated organic molecules 3,4,9,10-perylene-tetracarboxylic dianhydride, 1,4,5,8-naphthalene-tetracarboxylic dianhydride, and 1,8-naphthalene-dicarboxylic anhydride were investigated via gas phase and bulk ultraviolet photoemission spectroscopy and compared to density functional theory calculations. Values for final state effects such as intermolecular polarization were determined and
J J McFadden et al.
Biochemical and biophysical research communications, 168(1), 206-213 (1990-04-16)
In vitro metabolism of the herbicide bentazon was studied in microsomal membranes isolated from 6-day-old etiolated corn shoots. Microsomes isolated from shoots of nontreated seeds did not metabolize bentazon when assayed with NADPH or peroxides. However, microsomes isolated from shoots
C Gaillard et al.
FEBS letters, 352(2), 219-221 (1994-09-26)
In plants potentially toxic compounds are ultimately deposited in the large central vacuole. In this report we show that isolated barley mesophyll vacuoles take up the glucoside conjugate of the herbicide derivate [5-hydroxyphenyl]primisulfuron. Transport is stimulated by Mg-ATP and is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service