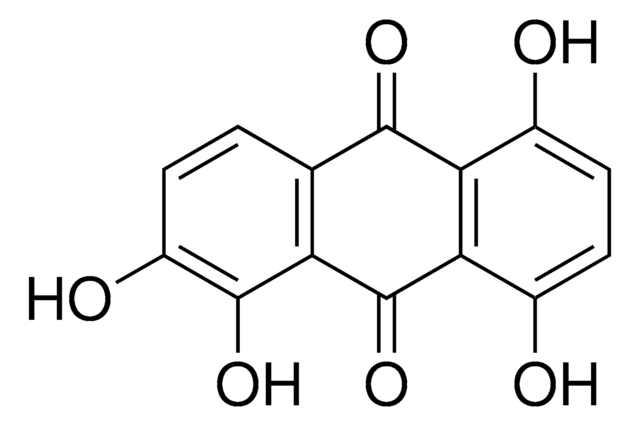
A89502
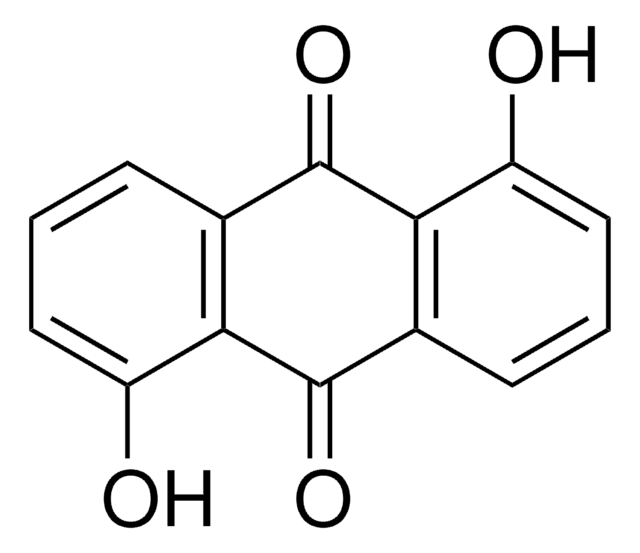
Anthraflavic acid
technical grade, 90%
Synonym(s):
2,6-Dihydroxyanthraquinone, Anthraflavine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H8O4
CAS Number:
Molecular Weight:
240.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
powder
mp
>320 °C (lit.)
SMILES string
Oc1ccc2C(=O)c3cc(O)ccc3C(=O)c2c1
InChI
1S/C14H8O4/c15-7-1-3-9-11(5-7)14(18)10-4-2-8(16)6-12(10)13(9)17/h1-6,15-16H
InChI key
APAJFZPFBHMFQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Anthraflavic acid can be used as a starting material to synthesize tetrahydroxy tetrathiafulvalene (TTF) derivatives, which are used as redox-active building blocks in supramolecular and materials science. It is also utilized to prepare phosphanylidene anthra[2,1-b]furans by reacting with dialkyl acetylenedicarboxylates and triphenylphosphine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A D Ayrton et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 26(11-12), 909-915 (1988-11-01)
Administration of the antimutagen anthraflavic acid to rats gave rise to significant increases in the hepatic microsomal O-deethylations of ethoxyresorufin and ethoxycoumarin, but not in the O-dealkylation of pentoxyresorufin nor in cytosolic glutathione S-transferase activity. Immunoblot studies of solubilized microsomes
A D Ayrton et al.
Biochimica et biophysica acta, 916(3), 328-331 (1987-12-18)
Consideration of the computer-optimised dimensions of anthraflavic acid indicates that it is essentially a planar molecule with a large area/depth ratio, that would preferentially interact with the polycyclic aromatic hydrocarbon-induced family of cytochrome P-450 proteins (cytochromes P-448). Anthraflavic acid was
Synthesis of tetrahydroxy-?-extended tetrathiafulvalenes as new supramolecular redox building blocks
Diaz Marta C, et al.
Tetrahedron Letters, 44(5), 945-948 (2003)
Regioselective synthesis of novel functionalized phosphanylidene anthra [2, 1-b] furan derivatives under solvent-free conditions
Nourmohammadian F and Gholami MD
Phosphorus, Sulfur, and Silicon and the Related Elements, 185(2), 340-346 (2010)
M Das et al.
Cancer research, 47(3), 767-773 (1987-02-01)
Naturally occurring plant phenols such as tannic acid, quercetin, myricetin, and anthraflavic acid are known to inhibit the mutagenicity of several bay-region diol-epoxides of polycyclic aromatic hydrocarbons (PAHs). The binding of bay-region diol-epoxides of PAHs to target tissue DNA is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service