All Photos(4)
About This Item
Empirical Formula (Hill Notation):
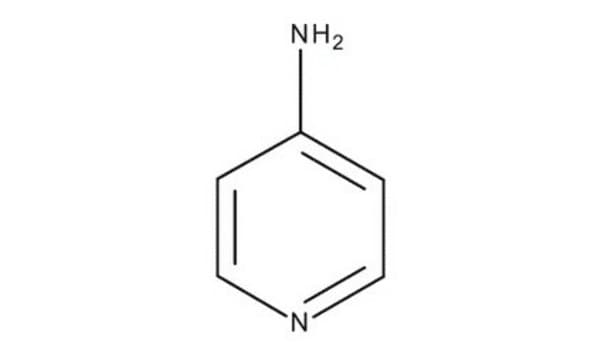
C5H6N2
CAS Number:
Molecular Weight:
94.11
Beilstein:
105692
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
flakes
bp
248 °C (lit.)
mp
60-63 °C (lit.)
SMILES string
Nc1cccnc1
InChI
1S/C5H6N2/c6-5-2-1-3-7-4-5/h1-4H,6H2
InChI key
CUYKNJBYIJFRCU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT RE 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pierre Garcia et al.
Organic letters, 13(8), 2030-2033 (2011-03-19)
Bimolecular cobalt-catalyzed [2 + 2 + 2] cycloadditions between yne-ynamides and nitriles afford bicyclic 3- or 4-aminopyridines in up to 100% yield. The high regioselectivity observed depends on the substitution pattern at the starting ynamide. Aminopyridines bearing TMS and Ts
E O'Hearn et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 17(22), 8828-8841 (1997-11-14)
Ibogaine, an indole alkaloid that causes hallucinations, tremor, and ataxia, produces cerebellar neurotoxicity in rats, manifested by degeneration of Purkinje cells aligned in narrow parasagittal bands that are coextensive with activated glial cells. Harmaline, a closely related alkaloid that excites
Jaslin Ikhsan et al.
Journal of colloid and interface science, 284(2), 383-392 (2005-03-23)
The sorption of 2-, 3-, and 4-aminopyridine on K-saturated Wyoming (SWy-K) and Texas (STx-K) and Ca-enriched Texas (STx-Ca) montmorillonite was measured at 25 degrees C with 10 mM KNO(3) or 3.3 mM Ca(NO(3))(2) as the background electrolyte. The aminopyridines adsorbed
J Pearce et al.
Journal of neurocytology, 15(2), 241-252 (1986-04-01)
The fine structure of identified neuromuscular synapses of the single excitatory axon to the distal accessory flexor muscle in lobster limbs was examined with freeze-fracture and serial thin-section electron microscopy. The latter technique reveals presynaptic dense bars with synaptic vesicles
Nitric oxide synthase inhibitor facilitates aminopyridine induced neocortical seizure.
B Boda et al.
Neurobiology (Budapest, Hungary), 4(1-2), 103-104 (1996-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service