924202
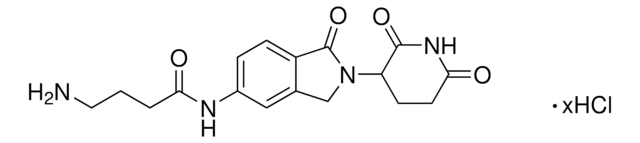
5-Amino-Thalidomide
≥95%
Synonym(s):
5-Amino-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione, C5 Pomalidomide, PROTAC® research ligand
About This Item
Recommended Products
ligand
thalidomide
Quality Level
Assay
≥95%
form
powder
SMILES string
O=C1C2=CC=C(N)C=C2C(=O)N1C3C(=O)NC(=O)CC3
InChI
1S/C13H11N3O4/c14-6-1-2-7-8(5-6)13(20)16(12(7)19)9-3-4-10(17)15-11(9)18/h1-2,5,9H,3-4,14H2,(H,15,17,18)
InChI key
IICWMVJMJVXCLY-UHFFFAOYSA-N
Application
of protein degraders with a C5 exit vector. This was previously listed under AMBH2D6FF88F.
Related Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Browse our growing synthesis and research tools: Protein Degrader Building Blocks
Other Notes
Efficient Synthesis of Immunomodulatory Drug Analogues Enables Exploration of Structure-Degradation Relationships
Targeting the C481S Ibrutinib-Resistance Mutation in Bruton′s Tyrosine Kinase Using PROTAC-Mediated Degradation
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Dermal - Repr. 1A
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service