901591
18-Crown-6 solution
1.0 M in THF
Synonym(s):
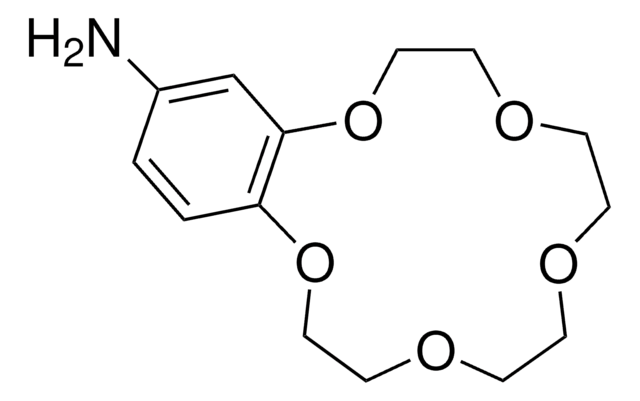
1,4,7,10,13,16-Hexaoxacyclooctadecane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
liquid
concentration
1.0 M in THF
density
0.944 g/mL
InChI
1S/C12H24O6/c1-2-14-5-6-16-9-10-18-12-11-17-8-7-15-4-3-13-1/h1-12H2
InChI key
XEZNGIUYQVAUSS-UHFFFAOYSA-N
Related Categories
Application
18-Crown-6 may be used to catalyze the N-alkylation of heterocyclic compounds and allylation of functionalized aldehydes.
Additive for greener etherification using KF-alumina.
Additive for greener etherification using KF-alumina.
Synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6
Synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6
Phase-transfer catalyst used in a chemoselective reduction of fused tetrazoles with NaBH4 and potassium hydroxide.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-2.0 °F
Flash Point(C)
-18.88 °C
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis, 3275-3275 (2006)
Phase-transfer alkylation of heterocycles in the presence of 18-crown-6 and potassium tert-butoxide.
Guida WC and Mathre DJ
The Journal of Organic Chemistry, 45(16), 3172-3176 (1980)
Preparation and purification of 18-crown-6 [1, 4, 7, 10, 13, 16-hexaoxacyclooctadecane].
Gokel GW, et al.
The Journal of Organic Chemistry, 39(16), 2445-2446 (1974)
Shih-Wey Yeh et al.
Inorganic chemistry, 51(7), 4076-4087 (2012-03-13)
The reversible redox transformations [(NO)(2)Fe(S(t)Bu)(2)](-) ⇌ [Fe(μ-S(t)Bu)(NO)(2)](2)(2-) ⇌ [Fe(μ-S(t)Bu)(NO)(2)](2)(-) ⇌ [Fe(μ-S(t)Bu)(NO)(2)](2) and [cation][(NO)(2)Fe(SEt)(2)] ⇌ [cation](2)[(NO)(2)Fe(SEt)(2)] (cation = K(+)-18-crown-6 ether) are demonstrated. The countercation of the {Fe(NO)(2)}(9) dinitrosyliron complexes (DNICs) functions to control the formation of the {Fe(NO)(2)}(10){Fe(NO)(2)}(10) dianionic reduced Roussin's
Sergey A Dergunov et al.
Journal of the American Chemical Society, 133(49), 19656-19659 (2011-11-15)
We describe a new co-entrapment and release motif based on the combination of noncovalent and steric interactions in materials with well-defined nanopores. Individual components enter hollow nanocapsules through nanopores in the capsule shell. Their complex, larger than the pore size
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service