756741
PCy3 Pd G2
95%
Synonym(s):
Chloro[(tricyclohexylphosphine)-2-(2′-aminobiphenyl)]palladium(II), Tricyclohexylphosphine Pd G2
About This Item
Recommended Products
Quality Level
Assay
95%
form
solid
feature
generation 2
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: C-H Activation
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
mp
244-246 °C
functional group
phosphine
SMILES string
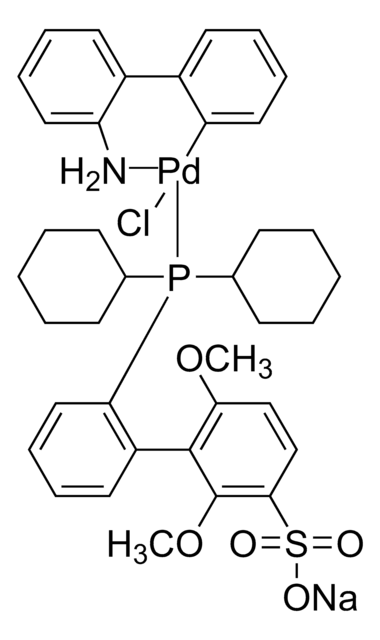
NC1=C(C2=C([Pd]Cl)C=CC=C2)C=CC=C1.C3(CCCCC3)P(C4CCCCC4)C5CCCCC5
InChI
1S/C18H33P.C12H10N.ClH.Pd/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;;/h16-18H,1-15H2;1-6,8-9H,13H2;1H;/q;;;+1/p-1
InChI key
CYJRABDADOSMKA-UHFFFAOYSA-M
Application
- To prepare diarylmethanes by Suzuki cross-coupling reaction with heterocyclic-chloromethyl derivatives with aryl/heteroaryl boronic acids.
- To synthesize poly(arylene)s by the Suzuki cross-coupling polymerization reaction between aryl dihalides and aryldiboronic acids.
- In the C-H bond functionalization reactions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service









![Mesyl[(tri-t-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)](/deepweb/assets/sigmaaldrich/product/structures/358/298/6539c19e-808c-4cd1-b9e8-19c6928f2384/640/6539c19e-808c-4cd1-b9e8-19c6928f2384.png)