673013
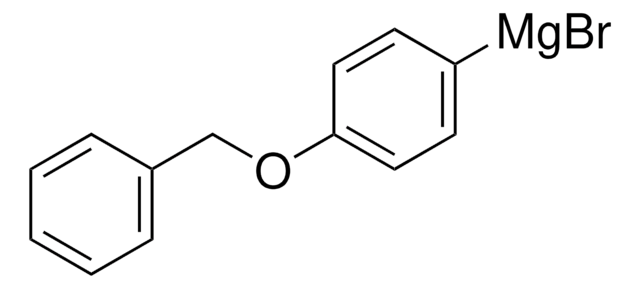
2-Benzyloxyphenylmagnesium bromide solution
1.0 M in THF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H11BrMgO
CAS Number:
Molecular Weight:
287.43
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
reaction type: Grignard Reaction
Quality Level
concentration
1.0 M in THF
density
1.047 g/mL at 25 °C
functional group
phenyl
SMILES string
Br[Mg]c1ccccc1OCc2ccccc2
InChI
1S/C13H11O.BrH.Mg/c1-3-7-12(8-4-1)11-14-13-9-5-2-6-10-13;;/h1-9H,11H2;1H;/q;;+1/p-1
InChI key
VELKJPFZDYXBDE-UHFFFAOYSA-M
Application
2-Benzyloxyphenylmagnesium bromide can be used:
- As a substrate in the iron-catalyzed coupling reaction of aryl Grignard reagents with unprotected bromophenols.
- As a substrate in the cross-coupling of Grignard reagents using diaryl titanates and ferric chloride.
- In one of the key synthetic steps for the total synthesis of an antibiotic caboxamycin.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-4.0 - 5.0 °F - closed cup
Flash Point(C)
-20 - -15 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Iron-catalyzed oxidative biaryl cross-couplings via mixed diaryl titanates: significant influence of the order of combining aryl Grignard reagents with titanate
Liu KM, et al.
Chemical Communications (Cambridge, England), 51(22), 4655-4658 (2015)
Iron-Catalyzed Room Temperature Cross-Couplings of Bromophenols with Aryl Grignard Reagents
Xu L, et al.
Advanced Synthesis & Catalysis, 361(23), 5421-5427 (2019)
Iron-Catalyzed Room Temperature Cross-Couplings of Bromophenols with Aryl Grignard Reagents
Xu L, et al.
advanced synthesis and catalysis, 361(23), 5421-5427 (2019)
Iron catalyzed oxidative assembly of N-heteroaryl and aryl metal reagents using oxygen as an oxidant
Liu KM, et al.
Chemical Communications (Cambridge, England), 51(6), 1124-1127 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service