597139
4-Iodobenzylamine hydrochloride
95%
Synonym(s):
(4-Iodophenyl)methanamine hydrochloride, p-Iodobenzylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CH2NH2 · HCl
CAS Number:
Molecular Weight:
269.51
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
299-303 °C (lit.)
functional group
amine
iodo
SMILES string
Cl[H].NCc1ccc(I)cc1
InChI
1S/C7H8IN.ClH/c8-7-3-1-6(5-9)2-4-7;/h1-4H,5,9H2;1H
InChI key
GBJMURRFWZREHE-UHFFFAOYSA-N
General description
4-Iodobenzylamine hydrochloride can be synthesized in three steps from 4-iodobenzoic acid.
Application
4-Iodobenzylamine hydrochloride can react with methyl 4-bromomethyl-3-methoxycarbonyl cinnamate in the presence of triethylamine to give the corresponding cyclic amide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Design, synthesis, and evaluation of isoindolinone-hydroxamic acid derivatives as histone deacetylase (HDAC) inhibitors"
Lee S, et al.
Bioorganic & Medicinal Chemistry, 17(27), 4895-4900 (2007)
Venkateswara R Narreddula et al.
The Analyst, 146(1), 156-169 (2020-10-31)
Ultraviolet-photodissociation (UVPD) mass spectrometry is an emerging analytical tool for structural elucidation of biomolecules including lipids. Gas phase UVPD of ionised fatty acids (FAs) can promote fragmentation that is diagnostic for molecular structure including the regiochemistry of carbon-carbon double bonds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service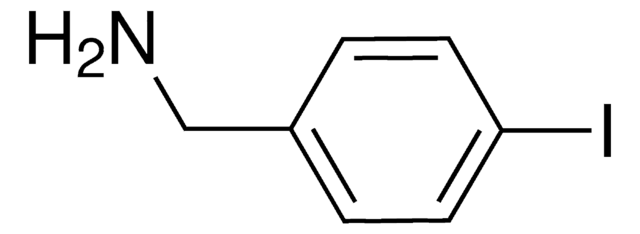








![Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)