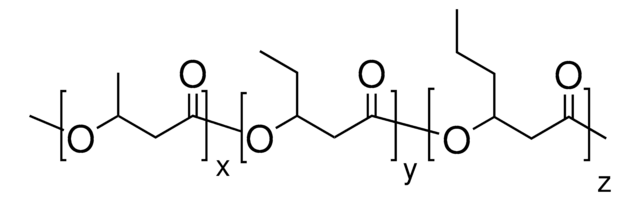
56655
(−)-Methyl (R)-3-hydroxyvalerate
≥98.0% (sum of enantiomers, GC)
Synonym(s):
(−)-Methyl (R)-3-hydroxypentanoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H12O3
CAS Number:
Molecular Weight:
132.16
Beilstein:
4655380
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (sum of enantiomers, GC)
optical activity
[α]20/D −37±3°, c = 1% in chloroform
refractive index
n20/D 1.427
bp
68-70 °C/5 mmHg (lit.)
density
1.029 g/mL at 20 °C (lit.)
functional group
ester
hydroxyl
SMILES string
CC[C@@H](O)CC(=O)OC
InChI
1S/C6H12O3/c1-3-5(7)4-6(8)9-2/h5,7H,3-4H2,1-2H3/t5-/m1/s1
InChI key
XHFXKKFVUDJSPJ-RXMQYKEDSA-N
Application
- Preparation of single-enantiomer 2-methyl-4-heptanol, a pheromone of Metamasius hemipterus, using (S)-2-methoxy-2-(1-naphthyl)propionic acid.: This research outlines the synthesis of a single-enantiomer pheromone compound using (S)-2-methoxy-2-(1-naphthyl)propionic acid. The process highlights the importance of enantiopure intermediates like (−)-Methyl (R)-3-hydroxyvalerate in the synthesis of biologically active compounds (Ichikawa and Ono, 2006).
Other Notes
Chiral building block for EPC syntheses; selective anti-alkylation of its dianion; OH protection and reduction of ester group to aldehyde or alcohol functions; stereoselective preparation of a dioxanone with pivalaldehyde
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G. Frater
Helvetica Chimica Acta, 62, 2825-2825 (1979)
P. DeShong et al.
Tetrahedron Letters, 27, 2091-2091 (1986)
K. Mori et al.
Liebigs Ann. Chem., 159-159 (1990)
K. Mori et al.
Tetrahedron, 41, 919-919 (1985)
J. Zimmermann et al.
Helvetica Chimica Acta, 70, 1104-1104 (1987)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

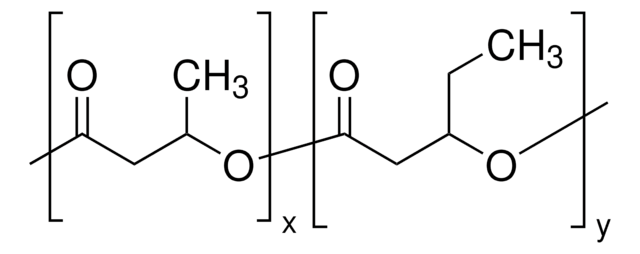
![Poly[(R)-3-hydroxybutyric acid] natural origin](/deepweb/assets/sigmaaldrich/product/structures/129/476/7d1c924b-f644-4889-a2d6-d7a923ce382c/640/7d1c924b-f644-4889-a2d6-d7a923ce382c.png)