561037
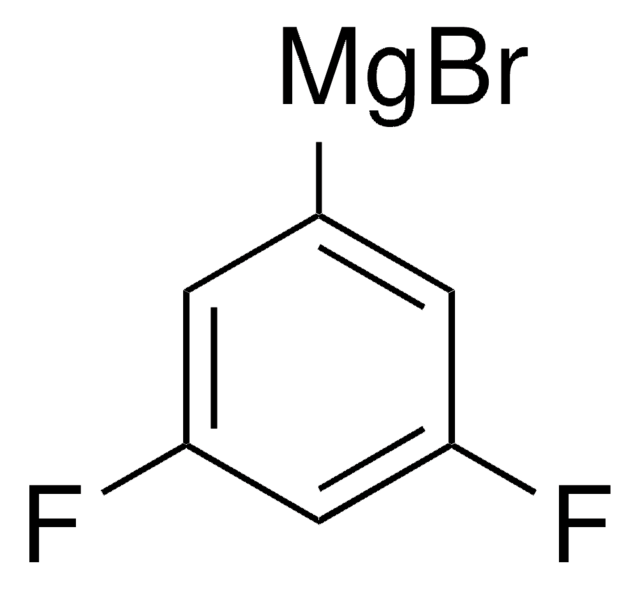
3,4-Difluorophenylmagnesium bromide solution
0.5 M in THF
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H3MgBr
CAS Number:
Molecular Weight:
217.29
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
reaction suitability
reaction type: Grignard Reaction
concentration
0.5 M in THF
bp
65 °C
density
0.965 g/mL at 25 °C
functional group
fluoro
storage temp.
2-8°C
SMILES string
Fc1ccc([Mg]Br)cc1F
InChI
1S/C6H3F2.BrH.Mg/c7-5-3-1-2-4-6(5)8;;/h1,3-4H;1H;/q;;+1/p-1
InChI key
WZHAVYGMKGPNFN-UHFFFAOYSA-M
Related Categories
Application
3,4-Difluorophenylmagnesium bromide can be used:
- To prepare 3,4-difluorophenylboronic acid, a key building block employed in the synthesis of norepinephrine transporter selective molecules.
- In the synthesis of trimethoxybenzophenone derived tubulin polymerization inhibitors.
- In one of the key synthetic steps for the preparation of diarylketoxime derivatives as potent MCH-1R antagonists.
- In the synthesis of biologically important hydroxybupropion analogs useful in smoking cessation.
Legal Information
Product of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Rieke is a registered trademark of Rieke Metals, Inc.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Target Organs
Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.0 °F - closed cup
Flash Point(C)
-17.2 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Discovery of novel diarylketoxime derivatives as selective and orally active melanin-concentrating hormone 1 receptor antagonists
Suzuki T, et al.
Bioorganic & medicinal chemistry letters, 19(18), 5339-5345 (2009)
Synthesis and monoamine transporter binding properties of 3α-(substituted phenyl) nortropane-2β-carboxylic acid methyl esters. Norepinephrine transporter selective compounds
Carroll FI, et al.
Journal of medicinal chemistry, 48(11), 3852-3857 (2005)
Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: aids to smoking cessation
Lukas RJ, et al.
Journal of Medicinal Chemistry, 53(12), 4731-4748 (2010)
2?Amino?3, 4, 5?Trimethoxybenzophenones as Potent Tubulin Polymerization Inhibitors.
Chuang H Y, et al.
ChemMedChem, 6(3), 450-456 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service