473790
trans-2-Phenylvinylboronic acid
97%
Synonym(s):
(E)-2-phenyl-Etheneboronic acid, (E)-Phenylethenylboronic acid, (E)-Styreneboronic acid, (E)-Styrylboronic acid, trans-(2-Phenylethenyl)boronic acid, trans-Phenylvinyl boronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
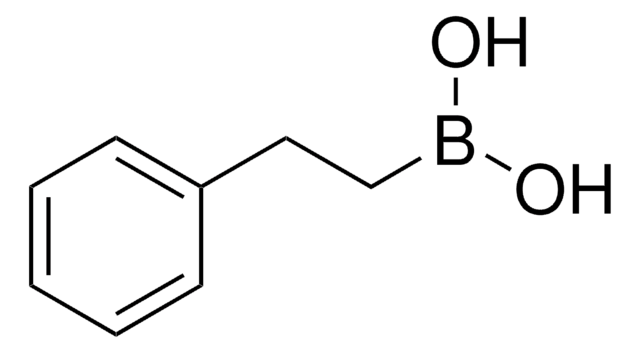
C6H5CH=CHB(OH)2
CAS Number:
Molecular Weight:
147.97
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
146-156 °C (lit.)
functional group
phenyl
SMILES string
OB(O)\C=C\c1ccccc1
InChI
1S/C8H9BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7,10-11H/b7-6+
InChI key
VKIJXFIYBAYHOE-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reagent used for
Reagent used in Preparation of
- Palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions
- Rhodium (Rh)-catalyzed intramolecular amination of aryl azides
- Diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction
- Copper (Cu)-mediated cyanation
- Rhodium (Rh)-catalyzed asymmetric addition
- Diastereoselective synthesis via iridium (Ir)-catalyzed addition
- Palladium (Pd)-catalyzed cascade cyclization
Reagent used in Preparation of
- Optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reaction
- Amino alcohol dienes via Petasis 3-component reaction using Ru-catalyzed ring-closing metathesis and isomerization
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Diastereoselective synthesis of tetrahydroquinolines via a palladium-catalyzed Heck-Suzuki cascade reaction
Wilson, J. E.
Tetrahedron Letters, 53, 2308-2311 (2012)
Rebecca L Greenaway et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(51), 14366-14370 (2011-11-25)
Cascade reactions: A modular assembly of azabicycles by using a cascade cyclization/Suzuki coupling/6π-electrocyclization of bromoenynamides is reported. The reaction offers a wide substituent scope on the bicyclic aminodiene products, which can be selectively oxidized as a general approach to aromatic
Ligand Effects on the Stereochemical Outcome of Suzuki-Miyaura Couplings
Lu, G-P.; et al.
The Journal of Organic Chemistry, 77, 370-3703 (2012)
Tomohiro Iwai et al.
Journal of the American Chemical Society, 134(2), 1268-1274 (2011-12-14)
Iridium complexes show high catalytic activity in intermolecular additions of acid chlorides to terminal alkynes to afford valuable (Z)-β-chloro-α,β-unsaturated ketones. Ligands in the catalytic system play a crucial role in this reaction. An N-heterocyclic carbene (NHC) is an efficient ligand
Xiangqing Feng et al.
Organic letters, 14(2), 624-627 (2012-01-12)
This paper describes a Rh(I)-catalyzed highly efficient and enantioselective 1,2-addition of arylboronic acids to α-diketones with the use of a simple sulfur-alkene hybrid ligand. With as low as a 0.1 mol % catalyst loading, a variety of optically active α-hydroxyketones
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)