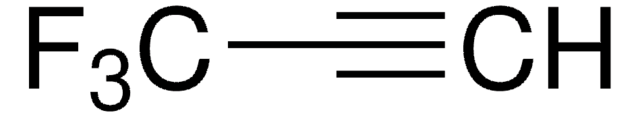All Photos(1)
About This Item
Linear Formula:
CF3C≡CCO2C2H5
CAS Number:
Molecular Weight:
166.10
Beilstein:
3539414
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.350 (lit.)
bp
96-98 °C (lit.)
density
1.162 g/mL at 25 °C (lit.)
functional group
ester
fluoro
SMILES string
CCOC(=O)C#CC(F)(F)F
InChI
1S/C6H5F3O2/c1-2-11-5(10)3-4-6(7,8)9/h2H2,1H3
InChI key
SFDRHPQGYUYYNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ethyl 4,4,4-trifluoro-2-butynoate is an unsymmetrical internal alkyne.
Application
Ethyl 4,4,4-trifluoro-2-butynoate has been used to investigate the regioselectivity of the insertion reaction with cyclometalated iridium and rhodium complexes.
It may be used in the synthesis of the following compounds :
It may be used in the synthesis of the following compounds :
- (2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(5-(ethoxycarbonyl)-6-(trifluoromethyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-2-yl)-6a,10b-dimethyl-4,10-dioxododecahydro-1H-benzo[f]isochromene-7-carboxylate
- (2S,4aR,6aR,7R,9S,10aS,10bR)-methyl 9-acetoxy-2-(6-(ethoxycarbonyl)-5-(trifluoromethyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-2-yl)-6a,10b-dimethyl-4,10-dioxododecahydro-1Hbenzo[f]isochromene-7-carboxylate
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reactivity and Regioselectivity of Insertion of Unsaturated Molecules into M- C (M= Ir, Rh) Bonds of Cyclometalated Complexes.
Li L, et al.
Organometallics, 29(20), 4593-4605 (2010)
Facile access to stereodefined dienoates and cyclopropylenoates containing a trifluoromethyl group.
Wang P-A, et al.
Journal of Fluorine Chemistry, 124(1), 93-97 (2003)
Anthony Lozama et al.
Journal of natural products, 74(4), 718-726 (2011-02-23)
As part of our continuing efforts toward more fully understanding the structure-activity relationships of the neoclerodane diterpene salvinorin A, we report the synthesis and biological characterization of unique cycloadducts through [4+2] Diels-Alder cycloaddition. Microwave-assisted methods were developed and successfully employed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service