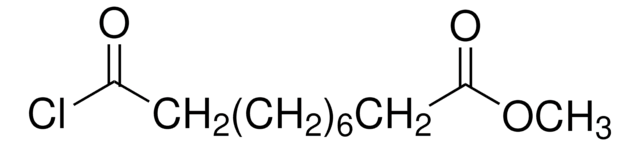385565
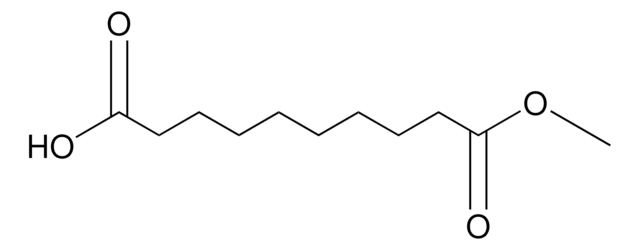
Methyl 8-chloro-8-oxooctanoate
96%
Synonym(s):
Methyl suberyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCO(CH2)6CO2CH3
CAS Number:
Molecular Weight:
206.67
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.45 (lit.)
density
1.456 g/mL at 25 °C (lit.)
functional group
acyl chloride
ester
SMILES string
COC(=O)CCCCCCC(Cl)=O
InChI
1S/C9H15ClO3/c1-13-9(12)7-5-3-2-4-6-8(10)11/h2-7H2,1H3
InChI key
RKUPOLBFJIEWBZ-UHFFFAOYSA-N
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A cyclodextrin-capped histone deacetylase inhibitor.
Amin et al.
Biochemical Medicine, 23(11), 3346-3348 (2013)
John Spencer et al.
ACS medicinal chemistry letters, 2(5), 358-362 (2011-05-17)
N(1)-Hydroxy-N(8)-ferrocenyloctanediamide, JAHA (7), an organometallic analogue of SAHA containing a ferrocenyl group as a phenyl bioisostere, displays nanomolar inhibition of class I HDACs, excellent selectivity over class IIa HDACs, and anticancer action in intact cells (IC(50) = 2.4 μM, MCF7
Synthesis and properties of PI polyamide?SAHA conjugate.
Ohtsuki et al.
Tetrahedron Letters, 50(52), 7288-7292 (2009)
Laura Forster et al.
Analytical and bioanalytical chemistry, 394(6), 1679-1685 (2009-06-02)
A fluorescent assay for the evaluation of inhibitors of fatty acid amide hydrolase (FAAH) is described. Microsomes from rat brain served as enzyme source. N-(2-Hydroxyethyl)-4-pyren-1-ylbutanamide was designed and synthesized as novel fluorogenic substrate. For substrate solubilization, Triton X-100 was employed.
Synthesis of 1-Aminocyclopropyl-Pentanoic and-Heptanoic Acid Derivatives.
Gensini et al.
Letters in Organic Chemistry, 5(5), 328-331 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service