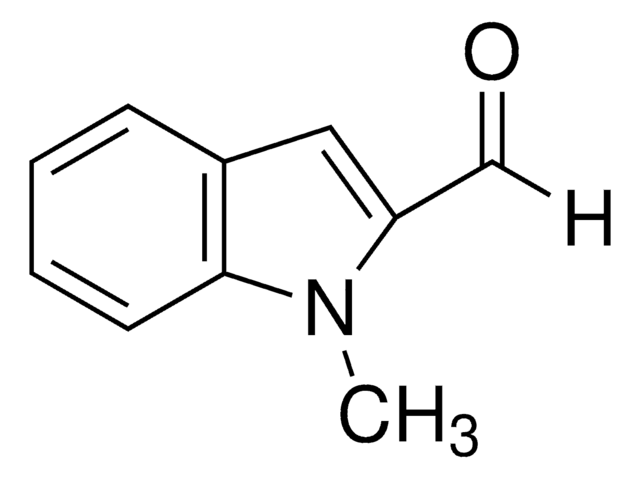
357987
1-Methylindole-3-carboxaldehyde
97%
Synonym(s):
3-Formyl-1-methylindole, NSC 83042
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9NO
CAS Number:
Molecular Weight:
159.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
70-72 °C (lit.)
functional group
aldehyde
SMILES string
Cn1cc(C=O)c2ccccc12
InChI
1S/C10H9NO/c1-11-6-8(7-12)9-4-2-3-5-10(9)11/h2-7H,1H3
InChI key
KXYBYRKRRGSZCX-UHFFFAOYSA-N
General description
1-Methylindole-3-carboxaldehyde is a heterocyclic indole aldehyde. 1-Methylindole-3-carboxaldehyde on condensation with 2-hydroxybenzohydrazide yields Schiff base.
Application
1-Methylindole-3-carboxaldehyde may be used in the synthesis of (Z)-3-(1-methyl-1H-indol-3-yl)-2-(thiophen-3-yl)acrylonitrile, via base-catalyzed condensation with thiophene-3-acetonitrile. It was also used in the preparation of monomer, required for the synthesis of poly(3-vinyl-1-methylindole).
- Reactant for preparation of nitroolefins and β-nitroalcohols via microwave- or ultrasound-assisted Henry reactions
- Reactant for synthesis of quinolinones via three-component Ugi reaction
- Reactant for synthesis of α-ketoamides as inhibitors of Dengue virus protease with antiviral activity in cell-culture
- Reactant for preparation of thiazolopyrimidinones as inhibitors of Bcl-2 proteins
- Reactant for preparation of vinylindoles via Peterson olefination or olefination with Nysted reagent
- Reactant for preparation of indolyl alkenes from microwave-enhanced Knoevenagel condensation as antibacterial agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Preliminary analysis of the 1H-and 13C-NMR spectra of poly (3-vinyl-1-methylindole).
Trumbo DL.
Polymer Bull., 37(1), 75-80 (1996)
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 60(Pt 3), o217-o218 (2004-03-09)
The title compound, C16H12N2S, has been synthesized by base-catalyzed condensation of 1-methylindole-3-carboxaldehyde with thiophene-3-acetonitrile. The product assumes an approximately planar Z configuration. The molecule has a thienyl-ring flip disorder.
Wagee A Yehye et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 9), o1824-o1824 (2008-01-01)
In the crystal structure of the title Schiff-base, C(20)H(21)N(3)O(4), the amino group forms an N-H⋯O hydrogen bond to the acetyl group of an adjacent mol-ecule, forming a zigzag chain. The 2-hydr-oxy group is inter-nally hydrogen bonded to the amido group
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service