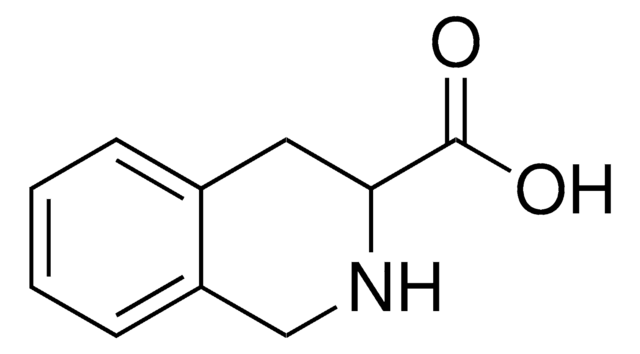
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H9NO2
CAS Number:
Molecular Weight:
163.17
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
optical activity
[α]20/D −114°, c = 1 in 1 M HCl
mp
177 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)[C@@H]1Cc2ccccc2N1
InChI
1S/C9H9NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-4,8,10H,5H2,(H,11,12)/t8-/m0/s1
InChI key
QNRXNRGSOJZINA-QMMMGPOBSA-N
Application
Catalyst for enantioselective cyclopropanations.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2 - Skin Sens. 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Roxanne K Kunz et al.
Journal of the American Chemical Society, 127(10), 3240-3241 (2005-03-10)
A new method for enantioselective organocatalytic cyclopropanation is described. This study outlines the identification of a new class of iminium catalyst based on the concept of directed electrostatic activation (DEA). This novel organocatalytic mechanism exploits dual activation of ylide and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service