333549
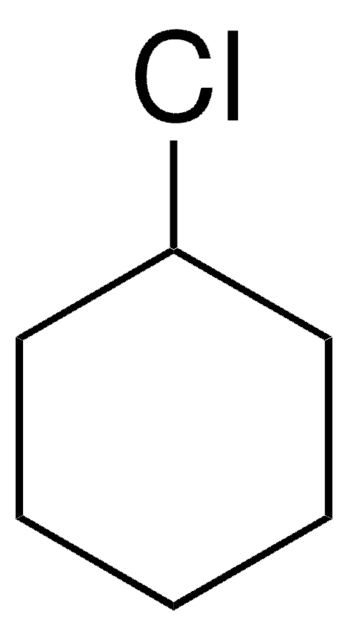
3-Bromocyclohexene
technical grade, 90%
Synonym(s):
2-Cyclohexen-1-yl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H9Br
CAS Number:
Molecular Weight:
161.04
Beilstein:
635953
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Assay
90%
form
liquid
refractive index
n20/D 1.528 (lit.)
bp
57-58 °C/12 mmHg (lit.)
density
1.4 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
−20°C
SMILES string
BrC1CCCC=C1
InChI
1S/C6H9Br/c7-6-4-2-1-3-5-6/h2,4,6H,1,3,5H2
InChI key
AJKDUJRRWLQXHM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Bromocyclohexene was used in the synthesis of N-tert-butoxycarbonyl-O-cyclohexyl-L-tyrosine. It was also used in the synthesis of enantiopure cyclohexitols such as muco-quercitol, D-chiro-inocitol and allo-inocitol.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y Nishiyama et al.
Chemical & pharmaceutical bulletin, 49(2), 233-235 (2001-02-24)
A facile and efficient synthesis of N-tert-butoxycarbonyl-O-cyclohexyl-L-tyrosine [Boc-Tyr(Chx)-OH] is described. Boc-Tyr-OH was treated with NaH in dimethylformamide and then with 3-bromocyclohexene to give N-Boc-O-(cyclohex-2-enyl)-L-tyrosine [Boc-Tyr(Che)-OH] in 70% yield. Hydrogenation of Boc-Tyr(Che)-OH over PtO2 afforded Boc-Tyr(Chx)-OH in almost quantitative yield. The
Synthesis of enantiopure cyclitols from (?)-3-bromocyclohexene mediated by intramolecular oxyselenenylation employing (S, S)-hydrobenzoin and (S)-mandelic acid as chiral sources.
Lee YJ, et al.
Tetrahedron, 61(8), 1987-2001 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service