292737
Cetyltrimethylammonium chloride solution
25 wt. % in H2O
Synonym(s):
Hexadecyltrimethylammonium chloride solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
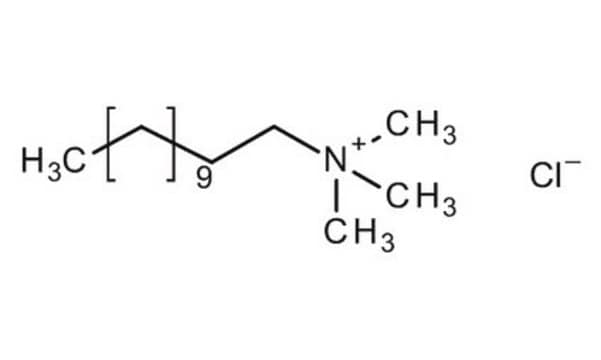
Linear Formula:
CH3(CH2)15N(Cl)(CH3)3
CAS Number:
Molecular Weight:
320.00
Beilstein:
3657974
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
concentration
25 wt. % in H2O
refractive index
n20/D 1.3778
density
0.968 g/mL at 25 °C
SMILES string
[Cl-].CCCCCCCCCCCCCCCC[N+](C)(C)C
InChI
1S/C19H42N.ClH/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(2,3)4;/h5-19H2,1-4H3;1H/q+1;/p-1
InChI key
WOWHHFRSBJGXCM-UHFFFAOYSA-M
Application
Cetyltrimethylammonium chloride (CTAC) is a cationic surfactant that can be used in the synthesis of mesoporous molecular sieves and micelle complexes. It can also be used as a dispersant to prevent aggregation of nanoparticles.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A non-enzymatic sensor based on Au@ Ag nanoparticles with good stability for sensitive detection of H2O2
Li Z, et al.
New. J. Chem., 40(3), 2115-2120 (2016)
Overgrowth of mesoporous MCM-41 on faujasite.
Kloetstra K R, et al.
Microporous Mater., 6(5-6), 287-293 (1996)
Growth of cetyltrimethylammonium chloride and acetate micelles with counterion concentration.
Ranganathan R, et al.
Journal of Colloid and Interface Science, 214(2), 238-242 (1999)
The surface tension of aqueous solutions of cetyltrimethylammonium cationic surfactants in presence of bromide and chloride counterions.
Para G, et al.
Colloids and Surfaces. A, Physicochemical and Engineering Aspects, 261(1-3), 65-73 (2005)
Qin Zhou et al.
Chemosphere, 90(9), 2461-2466 (2012-12-12)
The hexadecyltrimethylammonium bromide (HDTMAB) immobilized hollow mesoporous silica spheres were prepared for the efficient removal of perfluorooctane sulfonate (PFOS) from aqueous solution. Besides the traditional sorption behavior including sorption kinetics as well as effect of solution pH and temperature, the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service