281042
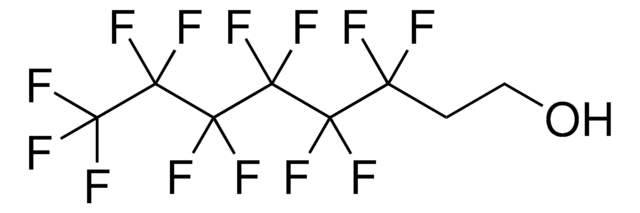
Tetradecafluorohexane
99%
Synonym(s):
Perfluorohexane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3(CF2)4CF3
CAS Number:
Molecular Weight:
338.04
Beilstein:
1802113
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.252 (lit.)
bp
58-60 °C (lit.)
mp
-74 °C (lit.)
density
1.669 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C6F14/c7-1(8,3(11,12)5(15,16)17)2(9,10)4(13,14)6(18,19)20
InChI key
ZJIJAJXFLBMLCK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetradecafluorohexane in the gas phase reacts spontaneously with lithium amalgam, to give a solid and intimate mixture of lithium fluoride and elemental polymeric carbon with a small amount of superstoichiometric lithium.
Application
Tetradecafluorohexane has been used:
- as a fluorocarbon organic solvent in the preparation of temperature-induced phase-separation solution
- to investigate boiling heat transfer mechanisms
- as a photosensitizer in fluorous biphasic singlet oxygenation
- as a novel reaction medium for photooxidation reactions
accessory
Product No.
Description
Pricing
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthetic Communications, 26, 1861-1861 (1996)
Journal of Heat Transfer, 118, 429-429 (1996)
Journal of the American Chemical Society, 118, 5312-5312 (1996)
Michael P Grubb et al.
Nature chemistry, 8(11), 1042-1046 (2016-10-22)
Spectroscopically observing the translational and rotational motion of solute molecules in liquid solutions is typically impeded by their interactions with the solvent, which conceal spectral detail through linewidth broadening. Here we show that unique insights into solute dynamics can be
Koichi Kitaguchi et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 30(6), 687-690 (2014-06-13)
A fluorocarbon and hydrocarbon organic solvent mixture is known as a temperature-induced phase-separation solution. When a mixed solution of tetradecafluorohexane as a fluorocarbon organic solvent and hexane as a hydrocarbon organic solvent (e.g., 71:29 volume ratio) was delivered in a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service