All Photos(3)
About This Item
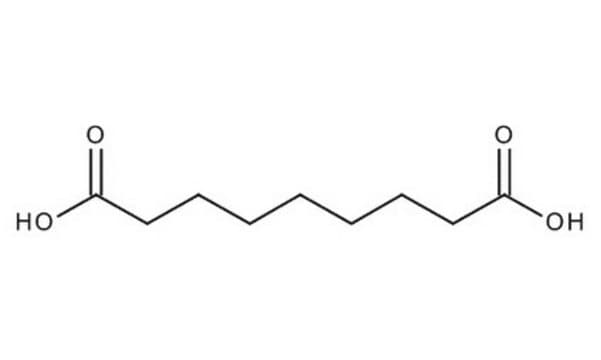
Linear Formula:
HO2C(CH2)7CO2H
CAS Number:
Molecular Weight:
188.22
Beilstein:
1101094
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor density
6.5 (vs air)
vapor pressure
<1 mmHg ( 20 °C)
Assay
98%
form
powder
bp
286 °C/100 mmHg (lit.)
mp
109-111 °C (lit.)
SMILES string
OC(=O)CCCCCCCC(O)=O
InChI
1S/C9H16O4/c10-8(11)6-4-2-1-3-5-7-9(12)13/h1-7H2,(H,10,11)(H,12,13)
InChI key
BDJRBEYXGGNYIS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Azelaic acid is an important monomer in the synthesis of various polymers. The presence of two carboxylic acid groups enables azelaic acid to act as a monomer for polycondensation reactions, leading to the formation of polyesters and other polymeric materials. Additionally, it is also used to prepare azelaic acid-based plasticizers to improve the mechanical properties of PVC, making them suitable for various applications, including films, coatings, and flexible products.
Application
Azelaic acid can be used:
- As a bio-based monomer in the development of biodegradable polymers. These polymers are explored for a range of applications, including packaging materials, agricultural films, and other products where biodegradability is a key requirement.
- As a crucial component in the synthesis of biodegradable copolyester plasticizers for PVC applications.
- As a monomer in the synthesizing poly(glycerol azelaic acid) for tissue engineering applications. Its contributions to biocompatibility, mechanical properties, degradation behavior, and hydrophilicity make it an essential building block for developing advanced biomaterials aimed at enhancing tissue regeneration processes. In drug delivery systems, azelaic acid may play a role in the synthesis of polymers or other compounds used in drug delivery, potentially enhancing medication effectiveness through improved solubility or stability.
- In skincare products.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
410.0 °F - closed cup
Flash Point(C)
210 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Seyed Dariush Taherzade et al.
Nanomaterials (Basel, Switzerland), 10(12) (2020-12-02)
Combined therapies emerge as an interesting tool to overcome limitations of traditional pharmacological treatments (efficiency, side effects). Among other materials, metal-organic frameworks (MOFs) offer versatilities for the accommodation of multiple and complementary active pharmaceutical ingredients (APIs): accessible large porosity, availability
Alvin B Coda et al.
Journal of the American Academy of Dermatology, 69(4), 570-577 (2013-07-23)
Excess cathelicidin and kallikrein 5 (KLK5) have been hypothesized to play a role in the pathophysiology of rosacea. We sought to evaluate the effects of azelaic acid (AzA) on these elements of the innate immune system. Gene expression and protease
Maria Zoeller et al.
Plant physiology, 160(1), 365-378 (2012-07-24)
Lipid peroxidation (LPO) is induced by a variety of abiotic and biotic stresses. Although LPO is involved in diverse signaling processes, little is known about the oxidation mechanisms and major lipid targets. A systematic lipidomics analysis of LPO in the
Vaneeta M Sheth et al.
Journal of the American Academy of Dermatology, 65(4), 699-714 (2011-09-17)
Several methods of treatment are available to patients with melasma. First-line therapy usually consists of topical compounds that affect the pigment production pathway, broad-spectrum photoprotection, and camouflage. Second-line therapy often consists of the addition of chemical peels, although these must
Nicola Castellucci et al.
Amino acids, 41(3), 609-620 (2011-04-14)
A small library of stereoisomeric pseudopeptides able to make gels in different solvents has been prepared and their attitude to make gels in the presence of several metal ions was evaluated. Four benzyl esters and four carboxylic acids, all containing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service