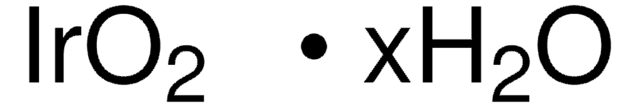208833
Ruthenium(IV) oxide hydrate
powder
Synonym(s):
Hydrous ruthenium oxide, Ruthenium dioxide hydrate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
RuO2 · xH2O
CAS Number:
Molecular Weight:
133.07 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: catalyst
core: ruthenium
SMILES string
O.O=[Ru]=O
InChI
1S/H2O.2O.Ru/h1H2;;;
InChI key
FGEKTVAHFDQHBU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Naiqiang Yan et al.
Environmental science & technology, 45(13), 5725-5730 (2011-06-15)
Catalytic conversion of elemental mercury (Hg(0)) to its oxidized form has been considered as an effective way to enhance mercury removal from coal-fired power plants. In order to make good use of the existing selective catalytic reduction of NO(x) (SCR)
Aleksandar R Zeradjanin et al.
ChemSusChem, 5(10), 1897-1904 (2012-08-16)
The reaction path of the Cl(2) evolution reaction (CER) was investigated by combining electrochemical and spectroscopic methods. It is shown that oxidation and reconstruction of the catalyst surface during CER is a consequence of the interaction between RuO(2) and water.
Hui Wang et al.
Journal of hazardous materials, 154(1-3), 44-50 (2007-11-13)
By using a self-made carbon/polytetrafluoroethylene (C/PTFE) O2-fed as the cathode and Ti/IrO2/RuO2 as the anode, the degradation of three organic compounds (phenol, 4-chlorophenol, and 2,4-dichlorophenol) was investigated in the diaphragm (with terylene as diaphragm material) electrolysis device by electrochemical oxidation
M Gonçalves et al.
Bioresource technology, 99(17), 8207-8211 (2008-05-02)
Electrochemical treatment of oleate using RuO2 and IrO2 type dimensionally stable anodes in alkaline medium was performed to develop a feasible anaerobic pre-treatment of fatty effluents. The results showed that the pre-treated solutions over RuO2 were faster degraded by anaerobic
Guolei Xiang et al.
Scientific reports, 2, 801-801 (2012-11-13)
Controls over the atomic dispersity and particle shape of noble metal catalysts are the major qualities determining their usability in industrial runs, but they are usually difficult to be simultaneously realized. Inspired from the Deacon catalyst in which RuO(2) can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service